Join Our Groups
TOPIC 4: FUELS AND ENERGY
A fuel is a substance that can be combusted or burnt to release energy as a byproduct. The energy can be in the form of heat, light, electricity, sound etc. This energy can be harnessed to power machines or used for other purposes such as heating or lighting. Combustion is the burning of fuel with energy released as a byproduct. Fuel is a very important substance for the existence of a modern man. Examples of fuels include petroleum products (petrol, diesel, fuel oil, kerosene, spirits, etc), natural gas, coal, wood, charcoal, producer gas, water gas, etc.
Fuel Sources
Different Sources of Fuels
Identify different sources of fuels
There are many types of substances that are used as fuels. The fuels exist as solids, liquids or gases. The most common substances that are used as fuels in Tanzania include wood, wood charcoal, coal, petroleum products and natural gas. These fuels are obtained from different sources as analysed below:
- Wood: wood is obtained from logs or poles of trees. The wood used as fuel in Tanzania is obtained from natural and artificial forests. Wood fuel is mainly used in rural areas where there are no alternative fuels. Wood is also a major source of fuel used by government institutions such as schools, colleges, hospitals, and military institutions.
- Charcoal: This fuel is made by heating certain substances such as wood and bones in a limited supply of air. Wood charcoal is the main source of fuel in urban areas and in some townships.
- Coal: coal used in Tanzania is mined at Kiwira coal mines. It is used indirectly for generating electricity or directly for powering machines in processing and manufacturing industries and factories. The electricity generated from coal is used in such industries as Tanga cement and several other industries in Dar es Salaam.
- Natural gas: This gaseous fuel is mined at Songosongo in Kilwa (Lindi region), located in southern Tanzania. The gas is used as a fuel at homes and in small industries. It is also used to generate electricity that is used in various manufacturing and processing industries. The electricity generated from this gas is also sold to Tanzania Electricity Supply Company (TANESCO) who distributes the energy to its various clients.
- Petroleum products (kerosene, diesel, petrol, fuel oil, fuel gas, etc.) These petroleum fractions are obtained from crude oil by the process of fractional distillation of crude oil (petroleum). Diesel, petrol and oil are used in vehicles and other machines. Kerosene is used in kerosene lamps and stoves for heating at homes and for other general purposes.
Methods of Obtaining Fuels from Locally Available Materials
Describe methods of obtaining fuels from locally available materials
Methods of making charcoal
When we heat certain organic matter in a limited supply of air, we obtain a black, solid residue called charcoal. The organic matter can be from plant or animal sources for example, wood or animal bones. Heating a substance in limited supply of air is called destructive distillation.
Wood or bone charcoal is made by the process of destructive distillation of wood or bones respectively. Charcoal is largely pure carbon. The entry of air during carbonization (destructive distillation) process is controlled so that the organic material does not burn down to ash as in conventional fire, but instead decompose to form charcoal.
Procedure for making wood charcoal
- Cut wood into small pieces.
- Arrange the wood pieces into a pile of wood on the ground.
- Cover the pieces of wood with soil, leaving one open space for setting fire.
- Set fire to the wood and then cover the open space with soil. Make sure that the wood is burning.
- After the wood is burned, uncover the soil and pull out the black solid substance underneath. This is the charcoal.
Coal formation
Coal is formed from the remains of lush vegetation that once grew in warm shallow coastal swamps. The following are the stages in the process of coal formation:
- The dead vegetation collects in the bottom of the swamp. It may start to decay. But decay soon stops, because the microbes that cause it need oxygen, and the oxygen dissolved in the stagnant, warm water is quickly depleted.
- The vegetation is buried under debris.
- Over hundreds of thousands of years, the environment changes. Seas flood the swamps. Heavy layers of sediment pile up on the dead vegetation, squeezing out gas and water and turning it intopeat.
- As the peat is buried deeper, the increasing heat and pressure compress it progressively to form different types of coal.
- As the process continues, the coal gets harder and more compact. Its carbon content also increases, giving different types of coal. Table bellow shows a summary of the stages in the process:
Stages of formation of different types of coal
| Name of coal | Carbon content | ||
| Peat | 60% | ||
| Pressure and Heat | Lignite | 70% | Hardness |
| Bituminous coal | 80% | ||
| Anthracite | 95% |
As carbon content increases so does energy given out per unit weight. But hard coal tends to have higher sulphur content, hence likely to cause environmental pollution. When burnt, the sulphur in the coal produces sulphur dioxide gas that is released into the atmosphere, causing air pollution. S(s)+O2(g)->S02(g)
Categories of Fuels
Fuels can be classified into three groups according to the physical state of the fuel. A fuel can be in any of the three states of matter namely, solid, liquid or gaseous state.
Fuels According to their States
Classify fuels according to their states
Solid fuels
Solid fuels include wood, charcoal, peat, lignite, coal, coke, etc. The immediate use of all these fuels is for heating and lighting. However, these fuels have a long history of industrial use. Coal was the fuel for the industrial revolution, from firing furnaces to running steam locomotives and trains. Wood was extensively used to run locomotives. Coal is still used for generation of power until now. For example, in Tanzania the coal mined at Kiwira is used for generation of electricity. Also Tanga Cement Company uses coal as a source of power to run machines for production of cement.
Wood is used as a solid fuel for cooking, heating or, occasionally, as a source of power in steam engines. The use of wood as a fuel source for home heating is as old as civilization itself. Wood fuel is still common throughout much of the world. It is the main source of energy in rural areas.
Wood charcoal yields a large amount of heat in proportion to its quantity than is obtained from a corresponding quantity of wood, and has a further advantage of being smokeless. Wood charcoal is often used for cooking and heating, in blacksmithing, etc.
Animal charcoal is used for sugar refining, water purification, purification of factory air and for removing colouring matter from solutions and from brown sugar. Animal charcoal is made by destructive distillation of animal bones.
Coke is a fuel of great industrial use. Coke is obtained by destructive distillation of coal. Most of the coke produced in industry is used as a reducing agent in the production of metals such as pig iron. A substantial amount of coke is also used for making industrial gases such as water gas and producer gas.
Coke is a better fuel than coal because when it is burning, it produces a clean and smokeless flame. When coal is used as a fuel, it produces many toxic gases during burning. Coke has high heat content and leaves very little ash.
Coal is a complex mixture of substances, and its composition varies from one place to another. It depends on coal's age and condition under which it was formed. Anthracite is a very hard black coal and it is the oldest of all types of coal.
When coal is heated in a limited supply of air, it decomposes. This thermal decomposition is called destructive distillation of coal. The products are coke, coal tar, ammoniacal liquor and coal gas.
Liquid fuels
Liquid fuels include petrol (gasoline) diesel, alcohol (spirit), kerosene (paraffin), liquid hydrogen, etc. Liquid fuels have advantage over solid fuels because they produce no solid ashes, and can be regulated by automatic devices. They are relatively more convenient to handle, store and transport than solid fuels.
Most liquid fuels in wide use are derived from fossils. Fossil fuels include coal, natural gas and petroleum. These fuels are formed from remains of sea plants and animals which lived millions of years ago. The remains became buried under layers of sediment. Immense heat and pressure resulted in the formation of coal gas and oil.
Energy produced when petroleum products (diesel, petrol, kerosene, natural gas etc) are burned, originated from the sun. This energy was transferred to animals through their consumption of plants or plant products. When the animals died, got buried, and compressed by heat and pressure, they produced oil which gives off that energy when burnt.
Petroleum fuels are used in cars and in various other machines. Fuels used in cars and lories (petrol and diesel), kerosene (for jet aircraft) and fuel oil (for ships), all came from crude oil. Some oil fuel is also used for electricity generation.
Ethanol burns with a clean, non-smoky flame, giving out quite a lot of heat. On a small scale, ethanol can be used as methylated spirit (ethanol mixed with methanol or other compounds) in spirit lamps and stoves. However, ethanol is such a useful fuel that some countries have developed it as a fuel for cars. In countries where ethanol can be produced cheaply, cars have been adapted to use a mixture of petrol and ethanol as fuel.
Brazil has a climate suitable for growing sugarcane. Ethanol produced by fermentation of sugarcane has been used as an alternative fuel to gasoline (petrol), or mixed with gasoline to produce "gasohol". Currently, about half of Brazil’s cars run on ethanol or "gasohol". "Gasohol" now accounts for 10% of the gasoline sales in the U.S.A.
The idea about the use of biofuel for fuelling automobiles and other machines has been borrowed by other countries including Tanzania. However, the programme has raised a bitter concern among different activists. Their doubt is that emphasis on growing crops for biofuel production may take up land that could otherwise be used for growing food crops. This, therefore, would mean that there would not be enough land to grow enough food to feed the ever-increasing human population. Hence, hunger will prevail. Notwithstanding all these shouting, biofuel crop production is there to stay!
Gaseous fuels
The use of gaseous fuels for domestic heating is common in urban areas. Compressed gas that is delivered to our homes in steel cylinders is liquefied propane, butane, or mixture of the two. When the valve is opened, the liquid gas vapourizes quickly into gas and passes through a pipe to the stove. Gaseous fuels are the most convenient fuels to handle, transport and store.
The following is a list of types of gaseous fuels:
- Fuel naturally found in nature: -natural gas -methane from coal mine
- Fuel gas from solid fuels or materials: -gas derived from coal (water gas and producer gas) -gas derived from wastes and biomass (biogas)
- Fuel gas made from petroleum.
Gaseous fuels used in industry
Producer gas and water gas are important industrial fuels.
Producer gas
Producer gas is produced by burning a solid carbonaceous fuel, such as coke, in a limited supply of air in a producer furnace. The reaction is exothermic and this makes coke to get hotter. Carbonaceous fuels are fuels that contain a high proportion of carbon. The producer gas is a mixture of carbon monoxide and nitrogen.
When air, mixed with a little steam, is passed through the inlet in the lower part of the furnace, the coke (carbon) combines with oxygen (from air) to form carbon dioxide:
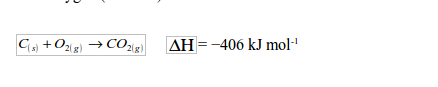
As the carbon dioxide formed rises up through the red-hot coke, it is reduced to carbon monoxide:

Since more heat (406 kJ) is produced in the lower part than is absorbed in the upper part of the furnace (163 kJ), some excess heat is obtained in the long run. This heat keeps the coke hot. The nitrogen gas in the air is not affected at all during the process. Hence, the overall reaction equation may be represented as follows:
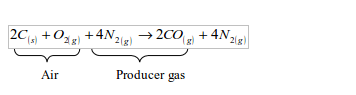
As a fuel, producer gas burns to give out carbon dioxide.

Because a good deal of producer gas contains nitrogen, a gas that does not support combustion, it has a lower calorific value compared to water gas. See table 4.2 for comparison.
Water gas
Water gas is produced by passing steam over white-hot coke at 1000°C. The gas is a mixture of hydrogen and carbon monoxide. The reaction is endothermic, causing the coke to cool.

Water gas burns as a fuel to give carbon dioxide and steam.
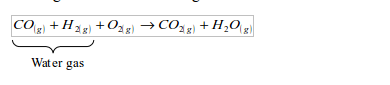
However, carbon monoxide is a very poisonous gas. The gas made from petroleum or coal contains some carbon monoxide, which makes it poisonous. Natural gas is safer and efficient, as it contains no carbon monoxide.
Characteristics of a good fuel
A good fuel burns easily to produce a large amount of energy. Fuels differ greatly in quality. There are certain characteristics, which make a good fuel. After all, there is no fuel among the different fuels known that posses all the virtues that a good fuel should have. Generally, a good fuel has the following
characteristics:
- It should be environmentally friendly (not harm the environment) in the course of its production and use, that is, it should not produce harmful or toxic products such as much smoke, carbon dioxide, carbon monoxide, sulphur dioxides, etc, which pollutes the air.
- It must be affordable to most people i.e. it must be cheap.
- It should not emit or produce dangerous by-products such as poisonous fumes, vapour or gases.
- It should have high calorific value i.e. it must burn easily and produce a tremendous quantity of heat energy per unit mass of the fuel.
- It should be easy and safe to transport, store, handle and use.
- It should be readily available in large quantities and easily accessible.
- It should have high pyrometric burning effect (highest temperature that can be reached by a burning fuel). Normally gaseous fuels have the highest pyrometric effect as compared to liquid and solid fuels.
- It should have a moderate velocity of combustion (the rate at which it burns) to ensure a steady and continuous supply of heat.
- A good fuel should have an average ignition point (temperature to which the fuel must be heated before it starts burning). A low ignition point is not good because it makes the fuel catch fire easily, which is hazardous, while high ignition point makes it difficult to start a fire with the fuel.
- A good fuel should have a low content of non-combustible material, which is left as ash or soot when the fuel burns. A high content of no-combustible material tends to lower the heat value of the fuel.
Calorific values of fuels
The heating value or calorific value of a substance, usually a fuel or food, is the amount of heat released during the combustion of a specific amount of it. The calorific value is a characteristic of each substance. It is measured in units of energy per unit of substance, usually mass, such as Kcal/Kg, J/g, KJ/Kg, KJ/Mol, MJ/m3, etc. Heating value is commonly determined by use of an instrument called bomb calorimeter.
By custom, the basic calorific value for solid and liquid fuels is the gross calorific value at constant volume, and for gaseous fuels, it is the gross calorific value at constant pressure.
Calorific values of solid, liquid and gaseous fuels
| Solid and liquid fuels | Calorific value (MJ/kg) |
| Alcohols | |
| Ethanol | 30 |
| Methanol | 23 |
| Coal and coal products | |
| Anthracite (4% water) | 36 |
| Coal tar fuels | 36 - 41 |
| General purpose coal (5-10% water) | 32 - 42 |
| High volatile coking coals (4% water) | 35 |
| Low temperature coke (15% water) | 26 |
| Medium-volatile coking coal (1% water) | 37 |
| Steam coal (1% water) | 36 |
| Peat | |
| Peat (20% water) | 16 |
| Petroleum and petroleum products | |
| Diesel fuel | 46 |
| Gas oil | 46 |
| Heavy fuel oil | 43 |
| Kerosene | 47 |
| Light distillate | 48 |
| Light fuel oil | 44 |
| Medium fuel oil | 43 |
| Petrol | 44.80 - 46.9 |
| Wood | |
| Wood (15% water) | 16 |
| Gaseous fuels at 15ºC, 101.325 kPa, dry | Calorific value (MJ/m3) |
| Coal gas coke oven (debenzolized) | 20 |
| Coal gas low temperature | 34 |
| Commercial butane | 118 |
| Commercial propane | 94 |
| North sea gas, natural | 39 |
| Producer gas coal | 6 |
| Producer gas coke | 5 |
| Water gas carburetted | 19 |
| Water gas blue | 11 |
Measuring the heat given out by fuels
We burn fuels to provide us with heat energy. The more heat a fuel gives out the better. The amount of heat given out when one mole of fuel burns is called heat of combustion. This is often written as
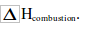
This value can be measured in the laboratory indirectly by burning the fuel to heat water. Simple apparatus is shown in figure bellow. The basic idea is: Heat gained by the
Heat gained by the water = heat given out by the fuel.
Method
These are the steps:
- Pour a measured volume of water into the tin. Since you know its volume you also know its mass (1 cm3 of water has a mass of 1g).
- Weigh the fuel and its container.
- Measure the temperature of the water.
- Light the fuel and let it burn for a few minutes.
- Measure the water temperature again, to find the increase.
- Reweigh the fuel and container to find how much fuel was burned.
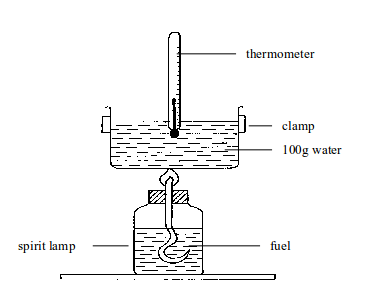
Measuring the energy value of a fuel
Calculations
It takes 4.2J of energy to raise the temperature of 1g of water by 1ºC. This constant value is called specific heat capacity of water, usually represented as 4.2Jg-1C-1 (4.2 joules per gram per centigrade). So, you can calculate the energy given out when the fuel burns by using this equation:
Energy given out = 4.2g-1C-1 mass of water (g) its rise in temperature (ºC).
Then since you know what mass of fuel you burned you can work out the energy that would be given out by burning one mole of it.
Example 1
The experiment gave these results for ethanol and butane. Make sure you understand the calculations:
Experimental results for heat determination
| Ethanol (burned in a spirit lamp) | Butane (burned in a butane cigarette lighter) |
| Results | Results |
| Mass of ethanol used: 0.9g | Mass of butane: 0.32g |
| Mass of water used: 200g | Mass of water used: 200g |
| Temperature rise: 20ºC | Temperature rise: 12ºC |
| Calculations | Calculations |
| Heat given out = or 16.8KJ | Heat given out = = 10080J or10.08KJ |
| The formula mass of ethanol is 46. 0.9g gives out 16.8KJ of energy. So, 46g gives out of energy | The formula mass of butane is 58. 0.32 gives out 10.08KJ of energy. So, 58g gives out KJ of energy |
| So, H combustion for ethanol is -859KJ/mol | So, H combustion for butane is -1827 KJ/mol |
Example 2
Determination of energy (calorific) value of ethanol
The energy/heating/calorific value of a fuel refers to the amount of heat given out when a specific amount of fuel is burned.
Experiment
Aim: To find out the energy value of ethanol.
Materials: water, beaker, thermometer, weighing balance, spirit lamp and ethanol.
Procedure:
- Pour a known volume of water into a beaker.
- Measure the temperature of the water.
- Fill the spirit lamp with enough ethanol.
- Weight the mass of both the ethanol and the lamp.
- Light the lamp and let it continue burning for a few minutes before putting it off.
- Measure the water temperature again, to find the increase.
- Reweigh the ethanol and its container to find how much ethanol was burned.
Record the following:
- Mass of spirit lamp + ethanol (initially)
- Mass of spirit lamp + ethanol (finally)
- Mass of ethanol burned
- Final temperature of water
- Initial temperature of water
- Rise in temperature of water
- Mass of water
The amount of heat (q) released by ethanol is given by:

Specimen calculation:
Mass of lamp and ethanol initially = 50g
Mass of lamp and ethanol finally = 49.5g
Mass of ethanol burned = 50.0 — 49.5 = 0.5g
Mass of water = 100g
Final temperature of water = 42ºC
Initial temperature of water = 20ºC
Rise in temperature = 42ºC – 20ºC = 22ºC
Specific heat capacity of water = 4.2 Jg-1C-1
Heat given out = Mass of water Xspecific heat capacity Xtemperature rise
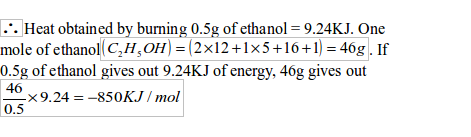
Repeat similar procedures with kerosene, charcoal, coal, firewood etc. and compare your results. Which fuel has more energy per gram? That is the most efficient fuel.
How reliable is the experiment?
The following table compares the experimental results with values from data book.
| Fuel | Heat of combustion in KJ/mol | |
| From the experiment | From a data book | |
| Ethanol | -859 | -1367 |
| Butane | -1827 | -2877 |
Note the big difference! The experimental results are almost 40% lower for both fuels. Why do you think there is such a big difference? There are two reasons for this:
- Heat loss: Not all the heat from the burning fuel is transferred to the water. Some is lost to the air, and some to the container that holds the fuel.
- Incomplete combustion: In case of a complete combustion, all the carbon in a fuel is converted to carbon dioxide. But here combustion is incomplete. Some carbon is deposited as soot on the bottom of the lamp and some converted to carbon monoxide. For example, when butane burns, a mixture of all these reactions may take place:
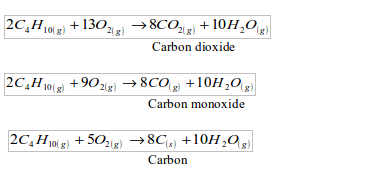
The less oxygen there is, the more carbon monoxide and carbon will form.
Renewable Energy Biogas
Renewable energy sources include biomass, geothermal energy, hydroelectric power, solar energy, wind energy, and chemical energy from wood and charcoal. These are called renewable energy sources because they are replenished within a short time. Day after day, the sun shines, wind blows, river flows and trees are planted. We use renewable energy sources mainly to generate electricity.
In Tanzania most of the energy comes from non-renewable sources. Coal, petroleum, natural gas, propane and uranium are examples of non-renewable energy sources. These fuels are used to generate electricity, heat our homes, move our cars and manufacture many kinds of products. These resources are called non-renewable because they cannot be replenished within a short time. They run out eventually. Once, for example, coal or petroleum is depleted, it may take millions of years to be replaced. So, these are non-renewable energy sources.
BIOGAS
Biogas is a gaseous fuel produced by the decomposition of organic matter (biomass). Under anaerobic conditions, bacteria feed on waste organic products, such as animal manure and straw, and make them decay. The product formed from this decay is called biogas, which consists mainly of methane, though other gases such as carbon dioxide, ammonia, etc, may also be produced in very small quantities. The biogas produced can be used as a fuel for cooking, heating, etc.
Raw materials for biogas production may be obtained from a variety of sources, which include livestock and poultry wastes, crop residues, food processing and paper wastes, and materials such as aquatic weeds, water hyacinth, filamentous algae, and seaweeds.
The Working Mechanism of Biogas Plant
Explain the working mechanism of biogas plant
The organic waste products are fed in a biogas plant. Prior to feeding the material into the plant, the raw material (domestic poultry wastes and manure) to water ratio should be adjusted to 1:1 i.e. 100 kg of excreta to 100 kg of water. Then adequate population of both the acid-forming and methanogenic bacteria are added.
The bacteria anaerobically feed on the liquid slurry in the digester. The major product of this microbial decomposition is biogas, which largely contain methane gas. The gas so produced is collected in the gas holder and then taped off. The gas is used as a fuel for cooking, heating and other general purposes.
The biological and chemical conditions necessary for biogas production
Domestic sewage and animal and poultry wastes are examples of the nitrogen-rich materials that provide nutrients for the growth and multiplication of the anaerobic organisms. On the other hand, nitrogen-poor materials like green grass, maize stovers, etc are rich in carbohydrates that are essential for gas production. However, excess availability of nitrogen leads to the formation of ammonia gas, the concentration of which inhibits further microbial growth. This can be corrected by dilution or adding just enough of the nitrogen-rich materials at the beginning.
In practice it is important to maintain, by weight, a C:N close to 30:1 for achieving an optimum rate of digestion. The C:N can be manipulated by combining materials low in carbon with those that are high in nitrogen, and vice versa.
A pH range for substantial anaerobic digestion is 6.0 – 8.0. Efficient digestion occurs at a pH near to neutral (pH 7.0). Low pH may be corrected by dilution or by addition of lime.
To ensure maximum digestion, stirring of the fermentation material is necessary. Agitation (stirring) can be done either mechanically with a plunger or by means of rotational spraying of fresh organic wastes. Agitation ensures exposure of new surfaces to bacterial action. It also promotes uniform dispersion of the organic materials throughout the fermentation liquor, thereby accelerating digestion.
A Model of Biogas Plant
Construct a model of biogas plant
The biogas plant consists of two components: the digester (or fermentation tank) and a gas holder. The digester is a cube-shaped or cylindrical waterproof container with an inlet into which the fermentable mixture is introduced in the form of liquid slurry. The gas holder is normally an airproof steel container that floats on the fermentation mix. By floating like a ball on the fermentation mix, the gas holder cuts off air to the digester (anaerobiosis) and collects the gas generated. As a safety measure, it is common to bury the digester in the ground or to use a green house covering.
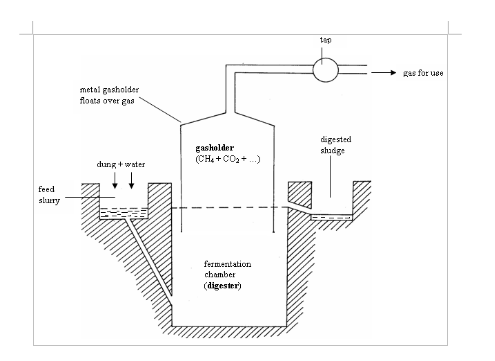
Structure of the biogas plant
The Use of Biogas in Environmental Conservation
Explain the use of biogas in environmental conservation
Environmental conservation is a major concern in life. We need to live in a clean and health environment so as to enjoy our lives better. The use of biogas as an alternative source of energy is essential in environmental conservation due to a number of reasons. These are some of the reasons:
- Biogas does not produce much smoke or ash, which could otherwise pollute the atmosphere or land. When the gas is burned it produces very little smoke and no ash as compared to other sources of fuel such as wood.
- The use of biogas for cooking and heating prevents the cutting down of trees to harvest firewood, or burn charcoal for fuel, a practice that could result to soil erosion, drought, etc. Hence, using the biogas as fuel helps to conserve the environment as no more cutting of trees may be done.
- Using cow dung, poultry manure and other excreta for biogas production helps keep the environment clean because these materials are put into alternative use instead of just being dumped on land, a fact that could lead to pollution of the environment.
- Some biomass employed in biogas production is toxic and harmful. By letting these materials be digested by bacteria, they may be turned into non-toxic materials that are harmless to humans, plants, animals and soil.
- The excreta used for production of biogas produce foul smell if not properly disposed of. Using this excrete to generate biogas means no more bad smell in air.
- Health hazards are associated with the use of sludge from untreated human excreta as fertilizer. In general, a digestion time of 14 days at 35ºC is effective in killing the enteric bacterial pathogens and the enteric group of viruses. In this context, therefore, biogas production would provide a public health benefit beyond that of any other treatment in managing the rural health and environment of developing countries.







EmoticonEmoticon