Join Our Groups
TOPIC 3: WATER
Occurrence and Nature of Water
The Occurrence and Nature of Water
Describe the occurrence and nature of water
Water is the most abundant liquid in nature. It is a compound of hydrogen and oxygen. It occurs on land as seas, oceans, rivers, springs, wells, etc. It also occurs in the atmosphere as rain, water vapour, clouds, etc. Water is the essential constituent of animal and plant life. Without water, no life could exist on earth. All living things need water to survive. About 60% of the human body by mass is made of water. A human being needs to drink about 2 litres of water per day to replace the water lost from the body via sweat, urine, breath, faeces, etc. If you did not replace this by eating and drinking, you would die in a matter of days.
Water is more important than food. A human being can survive without food for many weeks, but will die in a few days without water. So without water, no life can be sustained.
Water is the main constituent of the earth's surface. 70% of the earth's surface is covered by water. The remaining 30% is covered by land.
Types of water
There are four kinds of natural water namely, rain water, spring and well water, river water, and lake and sea water. Natural water is never pure. Water from difference natural sources contains substances dissolved in it.
Rain water
This is naturally distilled water. It is almost pure and it contains only gases and dust dissolved from the air. If the dissolved gases are acidic, e.g. sulphur dioxide, carbon dioxide or nitrogen dioxide, they may form "acid rain". In heavily industrialized countries where emission of these gases is very great, acid rains have been experienced. Rain water in non-industrial areas is fairly pure. It is safe to drink though it is tasteless. The taste in water is due to dissolved substances in it.
Spring and well water
When the rain falls, some water sinks into the ground to form ground water. This water percolates down the earth until it meets layers of impervious or impermeable (non-porous) rocks, which stop it from percolating or seeping any further. The ground water may reach the earth's surface as a spring. When a whole deep enough is dug to reach the ground water, a well results. Spring or well water is supposed to be clean, although it contains dissolved substances. As water passes through the earth, it is naturally filtered.
River water
River water contains dissolved and suspended solid materials. The water in some rivers is very muddy or sandy depending on the nature of the land from which the river originates and on which it flows. Most of the water we drink or use at home and industries is from rivers. To make the river water fit for use, all the substances dissolved and suspended in it must be removed or filtered.
Lake and sea water
Lakes and seas receive water from rivers. River water contains dissolved salts. As it flows through the land, some of its water evaporates into the air. When it reaches the sea or lake, more water still evaporates. As a result, sea and lake water will necessarily contain vast quantities of dissolved substances. Sea water contains about 3.6% by mass of the dissolved solids. Most of the dissolved solids compose largely of sodium chloride that can be obtained from sea water in large quantities. Three quarters of the ocean salts is sodium chloride (common salt).
The Water Cycle
Describe the water cycle
Water is always on move, travelling a never-ending, cyclical journey between the earth and the sky. This journey is referred to as the water cycle or hydrological cycle. The water cycle describes the continuous movement of water on, above and below the surface of the earth. During its movement, water is continuously reused and recycled. It also changes its physical state or form (liquid, vapour, and ice) at various stages in the water cycle. Figure 3.1 is a diagrammatic representation of the water cycle. It shows how the water moves around the earth's environment, changing its form through the process of evaporation, transpiration (loss of water from plants), condensation and precipitation (rainfall, snow, hail, fog, smog, etc.) Stages of the water cycle are described below:
- Heat from the sun causes water to evaporate from exposed water bodies such as oceans, seas, lakes, rivers dams, etc. This causes huge amounts of water vapour to float (laden) in the air. The vapour rises up. In the cooler upper parts of the atmosphere, the vapour cools and condenses to form tiny water droplets. The droplets form clouds.
- The clouds are drifted by wind. They cool further, and the droplets join to form larger drops of water which fall down as rain due to gravitation pull. On the other hand, if the air is very cold, they fall as hail, sleet or snow. The whole process is called precipitation.
- Some rain water soaks, and reappears as springs. Some flows over the ground as streams. The springs and streams feed rivers. The rivers flow to the ocean, sea or lake. The whole cycle starts again.
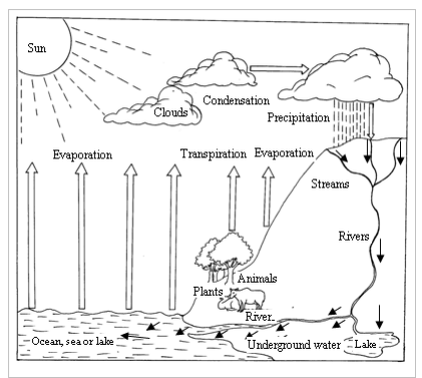
The water cycle
Water Cycle and Environmental Conservation
Relate water cycle to environmental conservation
Everyone understands why it is so important to keep our water clean. The fresh water that is available for use by people, plants and animals must be clean and safe.
Sometimes human carelessness pollutes the water system, loading harmful and unhealthy substances into the system at a rate that exceeds its natural restorative capabilities. When harmful substances are discarded (disposed off; dumped) into the environment, they may very well end up as part of the water cycle. An example of these acts may happen when untreated municipal and industrial wastes are directed into the water bodies such as rivers, lakes and seas. These substances are toxic and may harm human, marine, animal and plant life.
When chemicals are released into the air, they might well return to the earth with rain and snow or by simply settling. For example in industrial areas, sulphur dioxide dissolves in water from the clouds and with oxygen from the atmosphere to form sulphuric acid.
Sulphur dioxide + water + oxygen gives sulphuric acid = “acid rain”
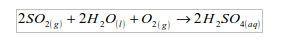
This then falls as "acid rain". The acid rain washes salts from the top soil. Acidic water and metal salts run into the lakes or rivers. The introduction of these new substances consequently increases the acidity and concentration of metal salts in the lake, river or stream. As a result, fish and other marine life die.
Nitrogen oxides, NOx, can also cause acid rain. When nitrogen dioxide gas reacts with water and oxygen in the atmosphere, the result is a weak solution of nitric acid.
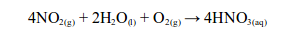
Carbon dioxide also reacts with water in the atmosphere to form a weak carbonic acid (rain water).
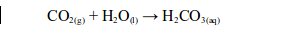
Pure water has a pH of 7.0. Normal rain is slightly acidic because of the carbon dioxide gas dissolved into it. It has a pH of about 5.5.
It has been confirmed that carbon dioxide (CO2), sulphur dioxide (SO2) and nitrogen oxides (NOx) are the primary causes of acid rain.
When harmful substances are dumped on land or buried in the ground, they might well find their way into ground water or surface water. These substances contaminate the water, which may be someone's or some community's drinking water.
Water plays an important role in the conservation of the environment and in determining human settlement and development. It also governs plant and animal distribution. Animals and plants, as components of the environment, are mainly concentrated in water or in areas where water is found.
Plant roots bind the soil particles together, making the soil compact and less susceptible to erosion. However, vegetation will only grow and flourish on land that receives sufficient rainfall. This is possible only if the water cycle is properly maintained by conserving natural forests and planting more trees to attract rainfall. So it is obvious that there is a strong relationship between rainfall (as a crucial stage of the water cycle) and the vegetation and soil (as components of the environment).
We use water from the lakes, rivers, wells or springs to irrigate crop and non-crop plants. So, when we distort the water cycle in some way or the other we may not have enough rainfall to fill up rivers or springs from which we obtain the water we use to conserve our environment (vegetation).
Properly watered soils support more plants. We all know that plants absorb carbon dioxide from the atmosphere, therefore, helping to purify the air naturally. In addition, plants produce oxygen gas, which is needed by all living organisms. If there is not enough rainfall, most plants will die, hence resulting to excessive accumulation of carbon dioxide, which may rise to toxic levels.
Excessive carbon dioxide in the atmosphere leads to intense heating of the earth's surface, a phenomenon described as global warming. The consequence of global warming include encroachment and extension of desert and arid lands, prolonged droughts, changes in rainfall patterns, etc.
These few facts show that there is a strong relationship and correlation between environmental conservation and the water cycle. Environmental degradation can lead to serious and irreparable aftermath to the water cycle.
Properties of Water
Properties of Water
Explain properties of water
Physical properties
Includes
- Extremely pure water is colourless, odourless and tasteless. The colour, taste or odours in water are due to dissolved impurities of organic and inorganic nature.
- Pure water is a very poor conductor of heat and electricity. However, water containing some dissolved inorganic impurities may conduct appreciably.
- Pure water freezes at 0ºC.
- Pure water boils at 100ºC at a pressure of 760 mmHg; and pure water will boil away completely with no change in temperature. Its melting point and boiling point are abnormally high due to hydrogen bonding.
- It is the only substance that occurs naturally in all the three states of matter – solid, liquid and gas.
- Water, as compared to other liquids, dissolves almost all substances, though in varying degrees of solubility. For this reason, water is usually called the universal solvent.
- It has a high surface tension than other liquids.
- It has a high specific heat index, which means that it can absorb a lot of heat before getting hot.
- It is miscible with many liquids, for example ethanol.
- The maximum density of pure water is 1 g cm-3 at 4ºC. When water is cooled gradually, it reaches its maximum density at 4 centigrade. The actual change from water to ice takes place at 0ºC.
- Pure water is neutral to litmus and has a pH of 7.0.
- Water expands when it freezes. Most substances contract when they change from liquid to solid state. Water is one of the very few substances that expand when they freeze. This behaviour is called anomalous expansion of water. Ice is therefore much less dense than water. The water molecules in the ice crystals are further apart from each other than in liquid water.
Chemical properties
Action of heat
Water is extremely stable to heat. A stable compound does not decompose easily by heating. It requires a very high temperature to decompose water. Water decomposes lightly at 2500ºC. It approaches complete decomposition at 5000ºC
Reaction with metals
The state in which water reacts with metals depends on the position of a metal in the electrochemical series as shown below:

It can be seen that water attacks metals differently depending on the metal’s position in the activity series. This is called the chemical activity series of metals.
Potassium
Potassium is vigorously attacked with cold water, producing hydrogen gas. The reaction of water with potassium is very violent and the hydrogen produced catches fire spontaneously with a lilac flame. The colour is due to the burning of small quantities of potassium vapour.
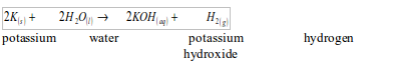
Sodium
The reaction of sodium with water is vigorous but the hydrogen liberated does not catch fire. Sodium reacts with cold water to produce hydrogen gas, which is detected by effervescence as the gas is liberated. If a flame is applied, it burns with a yellow flame (the yellow colour is from sodium).
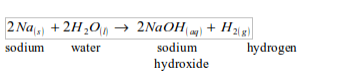
Calcium
Calcium reacts with water relatively slowly compared to sodium and potassium. The gas (hydrogen) given off explodes if mixed with air, and if a flame applied.
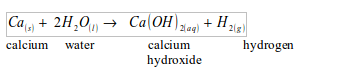
Magnesium
Magnesium reacts with steam to liberate hydrogen and magnesium oxide.

Zinc
If zinc is heated to redness in a current of steam, hydrogen is liberated.
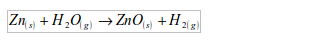
Iron
Iron does not react with cold water, but readily reacts with excess steam at red heat.
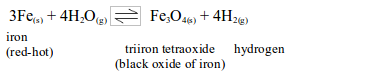
The above reaction can be made to proceed in the reverse direction by passing excess of hydrogen over heated triiron tetraoxide.
Reaction with non-metals
Carbon
Red-hot carbon reacts with steam at 1000ºC to give a mixture of carbon monoxide and hydrogen, known as water gas.
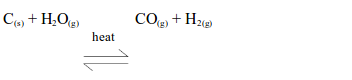
Red-hot carbon reacts with steam at 1000ºC to give a mixture of carbon monoxide and hydrogen, known as water gas.
Chlorine reacts with water to form a mixture of two acids.

Reaction with oxides
1. Water reacts with the oxides of most reactive metals to form hydroxides:
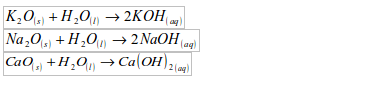
2. Water reacts with the oxides of some non–metals to form acids:

Formation of hydrates
Water combines with many salts to form hydrates. Different salt hydrates have different number of molecules of water of crystallization. The following are some examples:
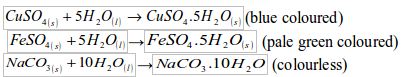
Synthesis of water
Water is a compound of hydrogen and oxygen. Its formula is H2O. You could make it in the laboratory by burning a jet of hydrogen in air. The reaction is fast and dangerous:
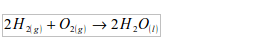
Hence, in the synthesis of water, hydrogen has to be prepared and then reacted with the oxygen of the air to give water. The apparatus used for water synthesis is as shown in figure 3.2 Hydrogen is produced by the reaction between zinc and cold dilute sulphuric acid
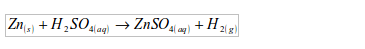
But the hydrogen to be used for synthesis of water has to be absolutely dry. This is achieved by passing it through anhydrous calcium chloride. It is then allowed to pass through the jet. When the hydrogen has displaced all the air in the apparatus, the gas is lit at the jet. The water forms as a gas. The gas condenses to liquid on an ice-cold tube. The burning hydrogen reacts with the oxygen of the air as given by the equation below:
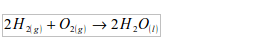
Physical tests for water
1. Water can be recognized by its action of turning white anhydrous copper (II) sulphate to blue.

The test, however, confirms the presence of water and not the absence of everything else except water. For example, a dilute sulphuric acid would turn anhydrous copper (II) sulphate from white to blue. That is why this test is called a physical test as opposed to a chemical test for water.
2. The presence of water can also be shown by the use of cobalt chloride paper. This is a filter paper impregnated with cobalt (II) chloride. The paper is blue in colour. The blue paper turns pink when in contact with water.
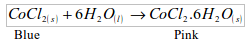
A chemical test for water
The two tests above only confirm the presence of water but do not indicate the purity of the water. Now, how can we test if an unknown colourless liquid contains water or if it is pure water? The presence of water will do the following:
- Will turn anhydrous copper (II) sulphate from white to blue.
- Will turn anhydrous cobalt (II) chloride from blue to pink.
To find out if a liquid is pure water, its boiling point or its freezing point must be measured. Pure water boils at exactly 100ºC and freezes at 0ºC at pressure of one atmosphere (760 mmHg). This is a chemical test for water.
Treatment and Purification of Water
Processes of Domestic Water Treatment and Purification
Perform processes of domestic water treatment and purification
Water for domestic use is chiefly obtained from rivers, springs and wells; and sometimes from lakes and seas. However, lake and sea waters may be to too salty for drinking or washing and hence not normally used for such purposes. But for some countries, the sea is a major source of drinking water. However, this water must be desalinized (have its salt removed) and purified before being used for drinking. The process is very expensive. It involves an expenditure of big sums of money. It is only practised in developed countries.
River and spring water must be boiled and filtered before drinking. At homes, water is normally boiled in big pans, cooled down, and then filtered by using a white, sterile and clean piece of cloth. The cloth is tied around the mouth of the container as shown in figure 3.3(a). As water is poured through the cloth, the particles in it are filtered off. The clean water is then poured in clay pots or plastic buckets and placed in a cool place, or put in a refrigerator to cool down ready for drinking.
Alternatively, boiled water can be filtered using a funnel as shown in figure (b) bellow, but you must ensure the gravel, sand and cotton wool used are thoroughly sterile. Sterilization can be achieved by soaking the gravel and sand in hot boiled water for quite some time.
The gravel traps any large floating substances. The coarse sand prevents small particles from passing through. The fine sand ensures even the small suspended particles do not pass through, while the cotton wool filters the very tiny particles.
(a): Filtering water using a piece of cloth
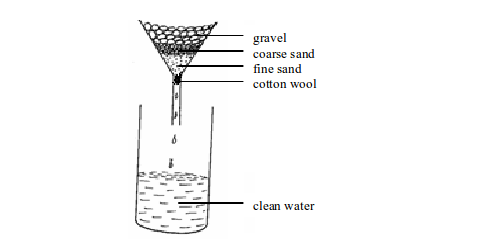
(b): Filtering water using a funnel
At home, water can also be purified with chemical purifiers. These chemicals are in liquid or tablet form. To purify water, a recommended amount of the purifier is added to a specific amount of water in a container. The water is shaken or stirred well. Then it is left to settle for at least 20 minutes before it can be safe for drinking and other domestic uses. To get the clearest water, it is advisable to filter the water thoroughly before adding the purifier. The commonest and most widely used purifiers are the waterguard and aquaguard.
In developed countries, commercial filters may be used to purify water at home. These filters contain charcoal or ceramic element that purifies the water as it passes through the filter.
The Processes of Urban Water Treatment
Describe the processes of urban water treatment
We obtain our water supply from surface water (for example, rivers, lakes and reservoirs) and ground water (for example, underground aquifers and lakes). Water from these sources is never completely pure, particularly if it is drawn from a river. The water may contain:
- bacteria – most are harmless, but some can cause diseases.
- dissolved substances – for example, calcium and magnesium compounds dissolved from rocks; and gases from the air.
- solid substances and debris – particles of mud, sand, grit, twigs, dead plants and perhaps tins and rags that people have dumped.
All these impurities are gathered by water as it passes through different parts of land as rivers or streams. Before water is safe to drink, the bacteria and solid substances must be removed.
Different towns and regions of the world apply different methods of water treatment. The more sophisticated and expensive methods are used by rich nations such as the UK and USA. Some steps in water treatment, however, are basic and used by all. They include the following:
Urban water treatment and purification
1. After water has been pumped through the screen to get rid of the larger bits of rubbish, it is pumped through a coarse filter which traps larger particles of solid. The filter could be beds of gravel and fine sand or anthracite.
2. In older purification plants, it may go to a sedimentation tank where chemicals are added to make smaller particles stick together. Then they sink to the bottom of the tank. Many chemicals could be used but the basic ones are the following:
(i) copper sulphate to remove algae;
(ii) sodium carbonate for softening; and
(iii) Aluminium sulphate in the form of potash alum, K2SO4.AL2(SO4)3.24H2O and slaked lime,Ca(OH)2are added for coagulating and precipitating all the suspended earthy material (clay matter). Bacteria and other microorganisms are captured by the coagulated mud, and precipitated. Sometimes instead of potash alum, iron (III) alum, (NH4)2.Fe(SO4)3.24H2Ocan be used.
The two chemicals (potash alum and slaked lime) react to form aluminium hydroxide and calcium sulphate:
The aluminium hydroxide is bulky and sticky. Therefore, bacteria, microorganisms and small particles can stick to it and get precipitated. The calcium sulphate is by far denser than water. Both the solid products (aluminium hydroxide, plus organic and inorganic particles stuck on it, and calcium sulphate) sink to the bottom of the tank. The whole process is called sedimentation.
3. Water from the sedimentation tank is passed through a fine filter. The filter could be made of layers of sand, gravel or carbon granules with thousands of tiny pores. The carbon removes coloured matter, odours (tastes) and noxious smells from the water. Filtration beds are expensive to install and require considerable labour to maintain.
4. After filtering, the water is chlorinated and may be aerated. Chlorine is added to kill harmful bacteria. Chlorine is such a useful disinfectant that it is used in swimming pools to kill bacteria. In aeration, water is pumped through fountains and sprout into the air. Aeration kills many dangerous aquatic bacteria. In some countries and regions, water is fluorinated by adding sodium fluoride to the water supply to help prevent tooth decay. Finally, the water is pumped to storage tanks, and then to homes and factories.
Importance of water treatment and purification
It is very important that community water supply be well treated and purified. There are several reasons for this practice. The following are some of the reasons:
- To kill harmful and disease-causing microorganisms such as bacteria, fungi, actinomycetes, amoeba, salmonella, etc.
- To remove toxic substances dissolved in water
- To remove solid substances and debris from the water such as tins, lags, plant remains, sand, algae, spirogyra, etc.
- To remove suspended earthy material (clay matter)
- To remove odour and unpleasant smells caused by different contaminants dissolved in water.
- To remove water hardness - sodium carbonate is added in water to remove both temporary and permanent hardness in water to make the water soft. Soft water forms lather easily with soap as compared to hard water which forms scum instead. This means that soft water requires less soap to form enough lather than hard water does. Therefore, soft water saves soap and hence money that could have been spent to purchase extra soap for washing.
- The sodium fluoride added to water in some areas helps to fight tooth decay.
Uses of Water
Uses of Water
State uses of water
Water is one of the most vital natural resources for all life on earth. The availability and quantity of water have always played an important part in determining not only where people can live, but also their quality of life. Even though there always has been plenty of fresh water on earth, water has not always been available when and where it is needed, nor is it always suitable for all uses. Water must be considered as a finite resource that has limits and boundaries to its availability and sustainability for use.
Where water supply is limited, conflicts may result between and among the various uses. The balance between supply and demand for water is a delicate one. The availability of usable water has and will continue to dictate where and to what extent development will occur. Water must be in sufficient supply for an area to develop, and an area cannot continue to develop if water demand far exceeds supply.
Water has numerous uses in life. The following are some of the uses of water:
- Biological use: Water is essential to life. Most of the reactions in animals and plants take place in solutions in water. Plants absorb minerals from the soil in solution form. Animals and plants are found near or in areas where water can be found.
- Domestic use: Domestic water use is probably the most important daily use of water for most people. It includes water that is used in the home every day including water for normal household purposes such as washing clothes and dishes, drinking, bathing, food preparation, flushing toilets, and watering lawns and gardens, etc.
- Industrial use: Water is a valuable resource to the nation’s industries for such purposes as processing, cleaning, transportation, dilution, and cooling in manufacturing industries. Major water-using industries include cloth, steel, chemical, paper, and petroleum refining. Industries often reuse the same water repeatedly for more than one purpose. Water is used as a solvent in many industrial processes. It is also used for cooling certain parts of machines.
- Irrigation: Water is artificially applied to farm, orchard pasture, and horticultural crops, as well as leaching of salts from the crop root zone in sodic soils. Non-agricultural activities include self-supplied water to irrigate public and private flower gardens, loans, football pitches, etc. Crop production in areas that receive little rainfall per year can be achieved through the practice of irrigation. Water for irrigation purposes can be drawn from rivers, lakes, swamps and even from seas.
- Water as a solvent: Water is regarded as a universal solvent. It dissolves almost all substances. For this reason, it is used for dissolution of chemicals ranging from poisonous chemicals used in agriculture to non-poisonous chemicals used in hospitals, laboratories, research stations and for other general purposes.
- Cooling and heating: Due to its high specific heat capacity, water is used as a coolant for cooling automobile engines and other machines. Hot water is used during winter for heating homes in temperate countries. In higher plants, evaporation causes a cooling effect and therefore helps to cool plant organs. During hot weather, some animals tend to wallow in water in order to cool their bodies either through evaporation or by water itself.
- Habitat: Water is a habitat for fish and all aquatic animals and plants.
- Livestock use: This includes water for stock animals, feedlots, dairies, fish farms and other non-farm animals. In arid regions of Tanzania, the Government has constructed dams to supply water to cattle, and for some domestic uses.
- Mining: Water is used in mines for extraction of naturally occurring minerals: solids, such as coal and ores; liquids, such as crude petroleum; and gases, such as natural gas. This includes quarrying, milling (such as crushing, screening, washing, and flotation), and other operations as part of mining activity.
- Generation of electricity: Hydroelectric power is generated by river water. Fast-moving river water (especially in waterfalls and cataracts) is used to turn turbines to generate hydroelectricity that is supplied to homes, industries, towns, etc. Most of the electricity we use at home is generated by this means. Only a small portion is generated through other means.
- Navigation and recreation: People, goods and services can be transported via water bodies like rivers, lakes and oceans by using vessels such as boats, dhows, canoes and ships. Water is also used for sports such as swimming, canoeing, fishing, yachting, water skiing, and many other sports carried out on, in and under the water.
The Solubility of Different Substances in Water and Organic Solvents
Compare the solubility of different substances in water and organic solvents
Water is a very good solvent for many ionic substances. There are few substances, which do not dissolve in water to some extent. Even when you drink a glass of water, you are also drinking a little of the glass as well. The amount is very small indeed, but for certain experiments ordinary glass vessels cannot be used as containers for water because of this solvent effect. Water is the commonest solvent in use, but other liquids, are also important. The other solvents are generally organic liquids such as ethanol, propanone, trichloroethane, etc. These organic solvents are also important because they will often dissolve substances that do not dissolve in water. The following table shows an example of substances that dissolve in water.
Substances soluble and insoluble in water
| Soluble compounds | Insoluble compounds |
| 1 All common sodium, potassium and ammonium salts | |
| 2. All common nitrates of metals | |
| 3. All common chlorides except………………….. | silver, mercury (I) and lead chloride |
| 4. All common sulphates except………………….. | lead, barium and calcium sulphates |
| 5. Sodium, potassium, and ammonium carbonates… | but other common carbonates are insoluble |
| 6. Sodium, potassium and ammonium hydroxides… | but other common hydroxides are insoluble. |
When salt is added to water and the mixture stirred, the salt dissolves. The product formed is termed as a solution. The solid that dissolves is known as a solute and the liquid (water) in which a solute dissolves is a solvent.
We can continue to add more salt and stir until no more salt dissolves. At this point, the water has dissolved the maximum amount of salt possible. The amount of salt dissolved denotes the maximum amount of salt which can normally be held in solution.
Adding a salt to water
The solution made is called saturated solution. The amount of the salt that has dissolved is called the solubility of the salt in water. The solubility of a substance is usually expressed as the mass of the substance dissolved in 100g of water. Solubility is sometimes expressed in moles of solute per dm3 of solution at that temperature.
To give a quantitative meaning to solubility, it is necessary to fix the amount of the solvent used and to state the temperature at which dissolution occurs. The amount of solvent is usually fixed at 100g. For example, the solubility of sugar (sucrose) at 20ºC is 240g in 100g of water. What is the maximum weight of sugar that will dissolve at 20ºC in a cup containing 350g of water? A saturated solution of a solute at a particular temperature is the one which will not dissolve any more of the solute at that temperature.
The solubility of a solute in water at a given temperature is the maximum amount of it that will dissolve in 100g of water at that temperature.
Dissolving a solid in water
Generally, the solubility of a solute increases with increase in temperature. However, there are a few exceptions e.g. the solubility of calcium hydroxide decreases with increase in temperature. Sugar dissolves very slowly in water at room temperature (20ºC). Stirring helps to make sugar dissolve more quickly. But if you keep on adding sugar to the water even with continuous stirring, eventually no more sugar will dissolve. Extra sugar sinks to the bottom. The solution is saturated.
Dissolving a solid in water at room temperature
Now let us look at what happens when you heat the sugar solution. If you heat the solution up to 20ºC there is still undissolved sugar at the bottom of the beaker. Increasing the temperature to 50ºC makes some sugar dissolve but there is still some left. But if the temperature is raised up to 80ºC all the sugar dissolves. You might even be able to dissolve more sugar!
Dissolving a solid in water at higher temperatures
Therefore, sugar is more soluble in hot than in cold water. In fact, this is usually the case with soluble solids. If a solid is soluble in a liquid, it usually gets more soluble as the temperature rises.
Solubility of different substances in different solvents
The solubility of a substance depends on the following factors:
1. The type of solvent used: Iodine is slightly soluble in water. Only 0.3g will dissolve in 100g of water at 20ºC. However, it is much more soluble in cyclohexane (organic solvent). 2.8g of iodine dissolve in 100g of cyclohexane at 20ºC
2. The particles in it: Let us consider the dissolution of sodium chloride in water. When dissolved in water, the salt dissolves to form Na+ and Cl- ions. If sodium chloride is added to water, the Na+ ions will be attracted to the slightly negatively charged oxygen atoms of the water molecules whereas Cl- ions will be attracted to the slightly positively charged hydrogen atoms of the water.
3. The temperature of the solvent: As we saw early, the temperature affects the solubility of substances, particularly solids. The higher the temperature the higher is the solubility.
If you shake some cyclohexane with a solution of iodine in water, almost all iodine leaves the water and moves into cyclohexane layer. So, cyclohexane is much better than water at separating iodine particles from each other. The iodine particles are more attracted to cyclohexane than they are to water. So, the solubility of each substance is different. Look at these examples:
| Compound | Mass (g) dissolving in 100g of water at 25ºC |
| Silver nitrate | 241.3 |
| Calcium nitrate | 102.1 |
| Magnesium chloride | 53.0 |
| Potassium nitrate | 37.9 |
| Potassium sulphate | 12.0 |
| Calcium hydroxide | 0.113 |
| Calcium carbonate | 0.0013 |
| Silver chloride | 0.0002 |
As you can see, one compound of a metal may be slightly soluble while another is almost soluble (compare silver nitrate and silver chloride). It depends on particles.
Measuring the solubility of a solid in water
Let us take potassium sulphate as our example. This is what to do:
- Put a weighed amount (say 2g) of potassium sulphate in a test tube. Add a little water from a measuring cylinder.
- Heat the test tube gently until the water is hot but not boiling. Add more water if necessary until the solid is just dissolved.
- Let the solution cool while stirring it with a thermometer. Note the temperature at which the first crystals form.
Measuring the solubility of a solid in water
Now look again at step 3. If you add a little more water, heat the solution again to make sure all the crystals have dissolved, and then let it cool, you will be able to find the solubility at a lower temperature. You can repeat this for a range of temperatures.
Calculating solubility
Since you know the mass of solute and the volume of water you used, you can work out the solubility as shown in the calculation below:
Example 1
2 grams of potassium sulphate were dissolved in 12.5 cm3 of water. On cooling, the first crystals appeared at 60ºC. What is the solubility of potassium sulphate in water at 60ºC?
Solution
12.5 cm3 of water weighs 12.5g. Also, remember that solubility is measured by 100g of water. If 2g of the salt dissolved in 12.5g of water, then the amount of the salt in 100g of water.
Therefore, the solubility of potassium sulphate in water at 60ºC is 16 grams.
Solubility of gases
Solid solutes usually get more soluble in water as the temperature rises. The opposite is true for gases. Table 3.3 shows the solubility of different gases in water at different temperatures.
Solubility of different gases in water
| Gas | Solubility (cm3 per 100cm3 of water) at..... | |||
| 0ºC | 20ºC | 40ºC | 60ºC | |
| Oxygen Carbon dioxide Sulphur dioxide Hydrogen chloride | 4.8171798050500 | 3.392.3425047400 | 2.556.6217044500 | 1.936.0-42000 |
Look at carbon dioxide. It is quite soluble in water at room temperature (20ºC). But when it is pumped into soft drinks under pressure, a lot more dissolves. Then when you open the bottle, it fizzes out of solution.
Look at hydrogen chloride. At room temperature, it is over 14000 times more soluble than oxygen.
Generally, the solubility of gases changes with temperature and pressure. It decreases with temperature and increases with pressure.
Solubility curves
The solubility of a particular solid in water can be measured over a range of temperatures up to 100ºC. The maximum mass of solid that will dissolve in 100g of water is found at each temperature. The values at each temperature can then be plotted to give a solubility curve. A curve that shows how the solubility of a substance changes with temperature is what we call a solubility curve.
Table bellow shows the solubility of some salts in water at different temperatures.
Solubility of some salts in water
| Temperature in ºC | Solubility in g of salt per 100g of water | ||
| Sodium chloride | Copper (II) sulphate | Potassium nitrate | |
| 10 | 38 | 18 | 20 |
| 20 | 38 | 20 | 30 |
| 30 | 38 | 24 | 44 |
| 40 | 38.5 | 28 | 60 |
| 50 | 38.5 | 34 | 80 |
| 60 | 39 | 42 | 104 |
| 70 | 39 | 50 | 152 |
For most substances, solubility in water increases with increase in temperature. Table above shows the solubility of some salts in water at different temperatures.
When the values for each salt shown on the table are represented on a graph paper, different solubility curves result.
Look at the values in table above again. On a graph paper, use the same set of axes to plot solubility (vertical axis) against temperature (horizontal axis). Draw a smooth best-fit curve for each salt.
- Which of the salts is the most soluble at 15ºC?
- Which of the three salts is the most soluble at 55ºC?
- At which temperature do sodium chloride and potassium nitrate have the same solubility?
The curves in figure bellow show how the solubility of different salts changes with temperature. You can see that the solubility of most solids increases with increase in temperature. The increase for sodium chloride is very small and almost negligible. The increase for the other salts is as shown in the graph.
Solubility curves for three solids in water (solubility measured in grams of solid per 100g of water)
For gases, the solubility decreases with increase in temperature. This means that decreasing the temperature will increase the solubility of gases. Figure 3.10 shows the solubility curves for some common gases. Compare these curves with those for solids in figure above.
The solubility of three gases from the air in water (solubility measured in grams of gas per 100g of water)
Using solubility curves
Data can be obtained from the solubility curves in various ways. For example, look at figure above.
(a) What mass of potassium nitrate dissolves in 100g of water at
- 40ºC and
- 50ºC?
From the graph:
- At 50ºC, 137.5g of potassium nitrate dissolve in 100g of water.
- At 40ºC, 62.5g of potassium nitrate dissolve in 100g of water.
(b) What mass of potassium nitrate will crystallize out when a saturated solution in 100g of water is cooled from 50ºC to 40ºC?







EmoticonEmoticon