Join Our Groups
TOPIC 6: TRANSFER OF THERMAL ENERGY

Conduction
The Concept of Conduction of Heat
Explain the concept of conduction of Heat
Conduction is the transfer of heat energy through solids in the manner which particles do not move from one point to another, for example in metals. Generally, solid substances contain particles which are close together. Each particle vibrates at one position but cannot move to another position.
The heated particles vibrate and collide with the other particles adjacent to them. In this way heat is transferred to the adjacent particles. The process goes on until the whole body get heated.
Good and Bad Conductors of Heat
Identify good and bad Conductors of Heat
Solid materials differ greatly in their ability to conduct HEAT.
Good conductors
These are the substances which allows the passage of heat energy easily example all metals.
Metals contain tiny particles called electrons (particles that carry electricity through metals) which are free to move inside the metal and carry energy from hotter places to colder places.
Bad conductors
These are materials which does not allow the passage of heat and electricity e.g Non – metals, woods.
| GOOD CONDUCTOR | BAD CONDUCTOR |
|
|
How to Minimize Heat Losses due to Conduction
Explain how to minimise Heat losses due to Conduction
There are some simple ways to reduce heat loss, including fitting carpets, curtains and draught excluders.
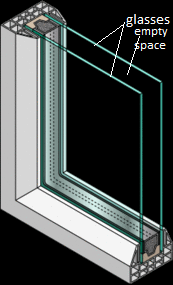
Heat loss through windows can be reduced using double glazing. The gap between the two panes of glass is filled with air. Heat loss through conduction is reduced, as air is a poor conductor of heat. Heat transfer by convection currents is also reduced by making the gap is very narrow.

A double glazed wall
Heat loss through walls can be reduced using cavity wall insulation. This involves blowing insulating an material into the gap between the brick and the inside wall, which reduces the heat loss by conduction. The material also prevents air circulating inside the cavity, therefore reducing heat loss by convection.

Cavity wall insulation
Knowledge of Conduction in Daily Life
Apply knowledge of conduction in daily life
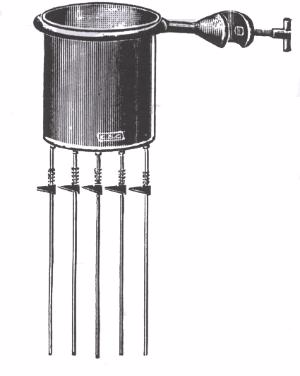
The difference in conductivity of various materials can be demonstrated using Edser’s apparatus

The apparatus consists of copper can with identical rods of aluminum, copper, lead and iron fixed to the bottom of the can. These identical rods are smeared/coated with wax.
The can is supported by a metal ring which is clamped to a retort stand. When hot water is poured inside the copper can, heat will be passed along the rods by conduction.
After some time, it will be observed that wax coated on the rods will melt and move down the rods. Note how far along the rods the wax has melted when the apparatus reaches a steady state.
This indicates that the materials from which the rods are made have different thermal conductivities. Of the four metal rods, the copper rod is observed to conduct heat more quickly than the rest.
Conduction of Heat Energy through Liquids
- All liquids expect mercury and gases are poor conductors of heat.
- Gases are far worse conductors of heat than liquids.
- Fluids are bad conductors of heat. They transfer heat by means of convection.
Convection
The Concept of Convection of Heat
Explain the concept of convection of heat
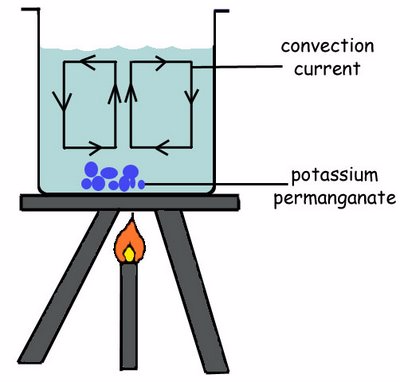
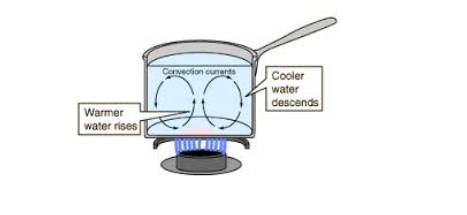
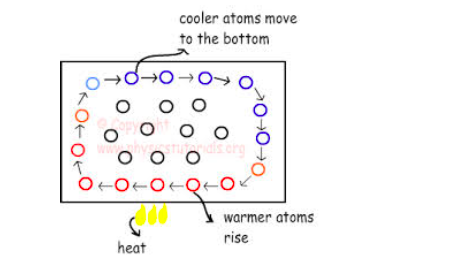
Convection is the transfer of heat energy through the fluids(Liquids or Gases) where by there is actual movement of particles. The heated particles move upwards due to decrease in their density and the cooler ones move downwards to get heated. These movements create the convectional currents.
Convection in Fluids in Terms of Kinetic Theory of Matter
Explain convection in fluids in terms of kinetic theory of matter
Convection currents are the currents of a liquid that move from the bottom to the top of the liquid container when the liquid is heated.
The heated liquid expands and becomes less denser and so can float upwards and replaced by colder denser liquids that sinks.
Convection in gases.
Convection air current occurs due to the unequal heating of the Earth’s atmosphere by the sun.
How to Minimize Heat Losses due to Convection in Daily Life
Explain how to minimise heat losses due to convection to daily life
When you understand the effects of cold water on the body, and how the body responds, you are far more prepared to make life-saving decisions, either for yourself or in a rescue situation.
It’s actually quite simple: the body attempts to maintain a constant core temperature (homeostasis) through a balance of heat loss and heat gain. Body heat is normally gained through activities such as exercise and shivering, and also with the application of external heat sources such as heat packs.
Convection is the process of air or water flowing by the skin and carrying away body heat. It’s convective heat loss that you try to prevent by staying as still as possible in the water. Staying still, the boundary layer of water next to the skin is heated by the body and remains undisturbed. If you move around in the water, you disrupt that boundary layer of warmer water, and that increases heat loss.
Once a body has been in cold water for an extended period of time, most of the skin is cool with little blood flow. However, there are critical areas that are lighter (warmer) than the surrounding tissue. This is because blood is flowing through major blood vessels, which are near the skin surface. These areas in the neck, armpits and groin are areas of high heat transfer. That means that these areas have high heat loss in the cold but allow heat gain in the heat. This is why, in a rescue scenario, the most effective rewarming often consists ofapplying external heat directly to the armpits as well as the chest.
As a final note, it’s important to realize that the activity of swimming (which is naturally thought of as producing a heat GAIN), in cold water conditions will result in increasing the blood flow to blood vessels close to the skin, and because of conduction and convection, it can actually increase the rate of heat LOSS and expedite the onset of hypothermia.
Knowledge of Convection to Daily Life
Apply knowledge of convection to daily life
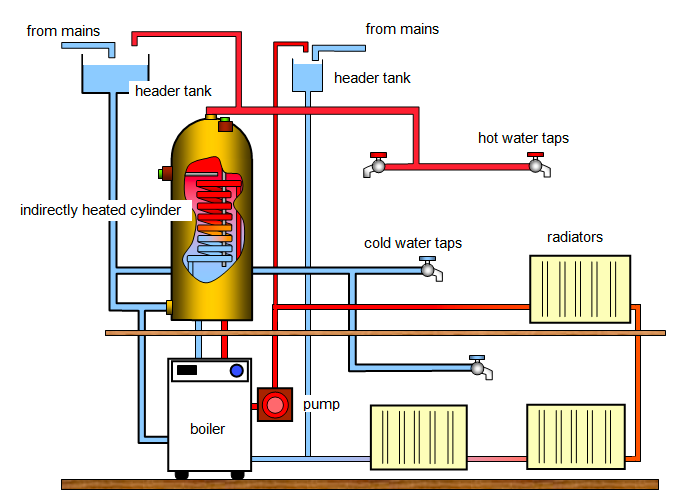
Domestic hot water system
- Convection currents are used to circulate hot water from a boiler in a domestic hot water system. The system consists of aboiler B, a hot water storage tank, H and cold water supply tank (cistern) C all connected by pipes.
- When water is heated (electrically or by fire) at the button of the boiler, it expands and become less dense, and so rises to the top.
- The hot water in the boiler passes through the outlets at the top of the boiler into the upper part of the hot water storage tank.
- The lower portion of the storage tank is filled with cold water from the cistern, which is high enough to drive the hot water out when the hot water tap T is open.
- The cistern is fitted with a ball-cock which maintains the level of water in the cistern by allowing water in when the level falls.
Radiation
The Concept of Radiation
Explain the concept of radiation
Radiation is transfer of heat energy from one point to another without the requirement of any material medium.
The stars including the sun illuminate the world by radiation.
Radiant energy from the sun reaches the Earth through the vast empty space (vacuum) existing between the atmosphere and the sun.
This energy travels with the speed of light and has similar properties to light i.e. Radiant energy can be reflected absorbed and Transmitted.
The body which absorbs radiant energy becomes heated up and its temperature rises.
Good Absorbers and Emitters of Radiant Heat
Identify good absorbers and emitters of radiant heat
Radiant energy can be detected by means of a thermopile.
Thermopile is an instrument which convents radiant energy ( radiant heat energy) into electrical energy.
If the terminals of the thermocouple are connect to a galvanometer by connecting wires, a current flows in the galvanometer G when the thermopile is directed towards a hot body, such as an electric lamp.
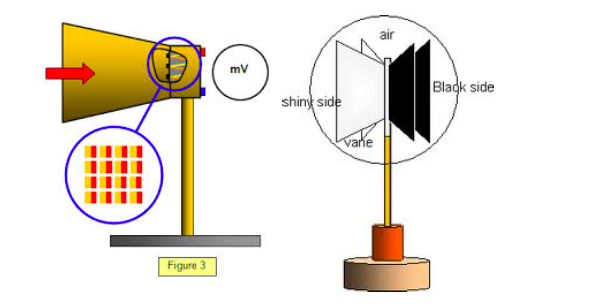
An increase in deflection of Galvanometer G is observed when the current thought the electric lamp is increased. Comparison of Radiant energy
The amount of Heat energy radiated by a body depends on:
- The Temperature of the body.
- The Nature of surface the body.
- The surface area for the body
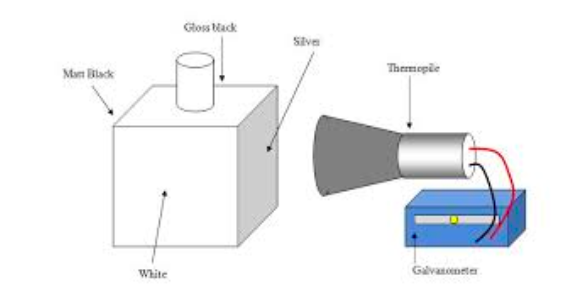
To demonstrate the fact that the amount of Heat energy radiated from a body depends on the nature and area of its surface (Leslie’s cube) can be used.
The figure below shows Comparison son of Radiant energy from different – substance.
- Leshe’s cube is a cube – shaped metal Box which has Three of its sides painted with different colours e.g Green, Black and Grey.
- One side is highly polished serve as a reflecting surface.
- The cube is placed on a Turn table R and Maintained Hot by Running steam into it.
- Thermopile, T connected to a galvanometer G is placed at a fixed distance from the cube by Turning the Turn table.
- The black side of the cube will produce the largest deflection of the Galvanometer G, While the polished surface will produces the leats deflection.
- The alternative demonstration of the absorption of radiant Heat by a surface can be per formed by using two tiny plates and Ban sern burner.
Heat Losses due to Radiation
Minimize heat losses due to radiation
Heat loss by radiation can be mad possible by the use materials which can reflect/send back thermal radiations. A good example is how the thermal flasks or vacuum flasks and hot pots can keep fluids and foods hot for a longer time.
The vacuum flask was designed by sir James Dewar for purpose of stoning condenser air in the liquid state.
Now days used for keeping liquids hot over a period of Time. It would also keep liquids Cold for a long time.
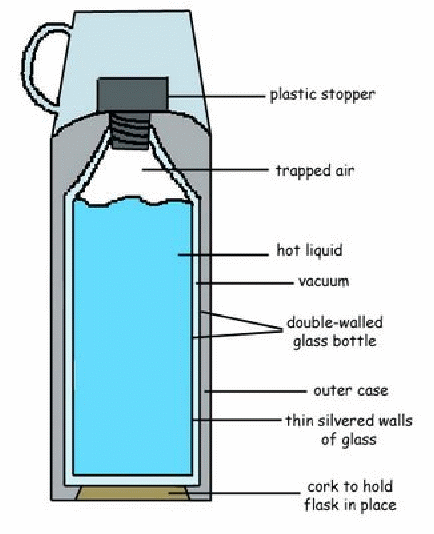
The vacuum flask consists of the double walled glass vessed with a vacuum between the walls.
The walls are silvered on the vacuum side. The flask controls convection, conduction and radiation of Heat energy.
Convection is prevented by the vacuum space between the walls and by closing the flask at the top.
Conduction is reduced by having the container made of glass, which is a bad conductor of heat. The stopper is made of a bad conductor e.g. cork or rubber.
The vacuum is also a non – conducting space. The outer glass wall is supported by a pad of felt or cork attached to a plastic case.
Radiation is minimized by the of silvered surfaces. The silvered surface reflects any Radiant heat energy coming from the outside or inside the flank.







EmoticonEmoticon