Join Our Groups
TOPIC 3: ACIDS, BASES AND SALTS
Acids and Bases
The Natural Sources of Acids and Bases
Investigate the natural sources of acids and bases
In everyday life, we deal with many substances that chemists classify as acids. For example, orange juice and grapefruit juice contain citric acid. These juices, and others of the like, contain ascorbic acid, a substance more commonly known as vitamin C. Examples of natural sources of acids and the type of acids they contain are shown in table below.
Some natural sources acids
| Source | Type of acid present |
| Mineral acids (HCl, H2SO4, HNO3, etc.) | Minerals |
| Tobacco | Salicylic acid |
| Tea | Tannic acid |
| Coffee | Chlorogenic acid |
| Sugar beet | Glutaric and adipic acids |
| Blackberry | Isocitric acid |
| Spinach, tomato | Oxalic acid |
| Sour (fermented milk) | Lactic acid |
| Bee, ant and nettle stings | Methanoic acid (formic acid) |
| Grapes, bananas, tamarinds | Tartaric acid |
| Citrus fruits | Citric acid ( lemons and limes have particularly high concentrations of citric acid; it can constitute as much as 8% of the dry weight of these fruits) |
Acids have a sour taste. Vinegar, lemon juice, grapefruit juice and spoilt or fermented milk are all sour tasting because of the presence of acids. The acids present in animal and plant materials are known as organic acids.
Salads are often flavoured with vinegar, which contains dilute acetic acid. Boric acid is a substance that is sometimes used to wash the eyes.
In any chemistry laboratory, we find acids such as hydrochloric acid (HCl), sulphuric acid (H2SO4), and nitric acid (HNO3). These acids are called mineral acids because they can be prepared from naturally occurring compounds called minerals. Mineral acids are generally stronger and should be handled with great care, especially the concentrated acids, for they are very corrosive. They can eat away metals, skin and clothing. Nevertheless, some acids are not corrosive even when they are concentrated. They are called weak acids. Ethanoic acid is one example. It is found in vinegar. In general, organic acids are weaker than natural acids.
You can tell if a substance is acid or not by its effect on litmus. Litmus is a purple dye. It can be used as a solution, or on paper, called litmus paper.Litmus solution is purple. Litmus paper for testing acids is blue while that for testing bases is red in colour. Acids will turn litmus solution red. They will also turn blue litmus paper red.
Bases do not usually occur naturally. So they are not normally obtained from natural sources. However, they are prepared in the laboratory or in industry. Bases can be classified into oxides, hydroxides or carbonates. Therefore, bases can be defined as the oxides, hydroxides or carbonates of metals. Bases taste bitter. A bitter taste is a characteristic of all bases.
Most bases are insoluble in water. The bases which dissolve in water are known as alkalis. The most common alkalis are potassium hydroxide (KOH), sodium hydroxide (NaOH), calcium hydroxide, Ca(OH)2, and ammonium hydroxide (NH4OH), also known as ammonia solution.
Alkalis turn litmus solution blue and red litmus paper blue.A substance, such as litmus, which changes from one colour to another when mixed with an acid or base, is called an indicator. Table 3.2 shows how acids and bases (alkalis) affect the colours of different indicators. We can use this clue of colour changes to tell whether an unknown substance is an acid or base (alkali).
Some common indicator colour changes
| Indicator | Colour in acid | Colour in alkali (base) |
| Methyl orange | orange | yellow |
| Phenolphthalein | colourless | Pink |
| Litmus | red | blue |
| Bromothymol blue | yellow | blue |
The Reactions of Acids with Various Materials
Determine the reactions of acids with various materials
Acids react with different substances to produce different products. These reactions are best carried out by using dilute acid solutions. The following are some reactions of dilute acids with various substances.
Reaction with metals
Acids react with quite reactive metals (not very reactive ones) to produce salt and liberate a hydrogen gas.
metal + acid → salt + hydrogen
It is unsafe to try this reaction with very reactive metals such as sodium or calcium. The reaction with such metals is so violent. Metals less reactive than lead, such as silver and gold have no reaction with dilute acids. Even with lead, it is difficult to see any reaction in a short time.
The salt produced when a dilute acid reacts with a metal depends on the acid and a metal used:
- Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g)
- Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Reaction with carbonates
Acids react with carbonates to give salt, water and carbon dioxide. In general, all carbonates give off carbon dioxide when they react with acids.
acid + metal carbonate → salt + water + carbon dioxide
The normal methods of preparing carbon dioxide in the laboratory are based on this reaction. Dilute hydrochloric acid is reacted with marble chips (calcium carbonates):
2HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)
Reaction with oxides and hydroxides (alkalis)
Hydroxides: acids react with alkalis, forming salt and water:
NaOH(aq) + HNO3(aq) → CaCl(aq) + H2O(l)
Oxides: They also react with metals oxides, forming salt and water:
ZnO(s) + 2HCl(aq) → ZnCl2(aq) + H2O(l)
The bases (oxides, hydroxides) all react in the same way with acids, and in the process, salts are formed. This type of reaction is known as neutralization reaction. It can be summarized up in a general equation:
acid + base → salt + water
Reaction with hydrogencarbonates (bicarbonates)
Acids react with hydrogencarbonates, forming salt, water and liberating carbon dioxide gas:
NaHCO3(aq) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g)
The Reactions of Alkalis with Various Materials
Determine the reactions of alkalis with various materials
Alkalis react with acids to produce salt and water. All alkalis, except ammonia solution, will react with ammonium compounds liberating ammonia gas. Aqueous solutions of alkalis will precipitate the insoluble hydroxides of other metals from the solutions of metal salts. Caustic alkalis attack aluminium, zinc and lead to form salts. They react with carbon dioxide to form carbonates.
The Reactions of Bases with Various Substances
Determine the reactions of bases with various substances
Characteristic reactions of bases
Dissolution in water
Most bases are insoluble in water. Some are soluble in water. Soluble bases are known as alkalis. The commonest alkalis are sodium hydroxide, (NaOH), calcium hydroxide, Ca(OH)2, potassium hydroxide, (KOH), and ammonium hydroxide or ammonia solution, (NH4OH). All alkaline solutions contain hydroxyl ions, OH-. In sodium hydroxide solution, the ions are produced like this:
NaOH(aq)→ Na+(aq)+ OH-(aq)
Like acids, alkalis can also be classified as strong or weak. Ammonia solution is a weak base because it ionizes just partially:
The rest of the bases are strong bases because they ionize fully into ions in solution.
Reaction with acids
Bases react with acids to produce salt and water. Refer to the reactions of acids with oxides and hydroxides discussed early.
Reaction with ammonium compounds
Alkalis turn litmus solution blue and red litmus paper blue.A substance, such as litmus, which changes from one colour to another when mixed with an acid or base, is called an indicator. Table 3.2 shows how acids and bases (alkalis) affect the colours of different indicators. We can use this clue of colour changes to tell whether an unknown substance is an acid or base (alkali).
Reaction with aqueous salts of metals
Aqueous solutions of alkalis will precipitate the insoluble hydroxides of other metals from the solutions of metal salts. Only NH4OH, KOH and NaOH are soluble enough in water.
All other hydroxides are insoluble and can be precipitated from aqueous solution by these three alkalis.
- When sodium hydroxide solution is added to copper (II) sulphate solution, a pale blue precipitate of copper (II) hydroxide is formed. CuSO4(aq) + 2NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
- Another example is the reaction between potassium hydroxide and iron (II) chloride, which precipitates iron (II) hydroxide. FeCl3(aq) + 3KOH(aq) → Fe(OH)3(s) + 3KCl(aq)
Reaction with metals
Caustic alkalis attack very few metals. The metals known to be attacked by the alkalis are aluminum, zinc and lead, where the aluminate, zincate and plumbate (II) are formed respectively. The aluminum will react thus:2Al(s) + 6NaOH(aq) + 6H2O(l) → 2Na3Al(OH)6(aq) + 3H2(g)(sodium aluminate)
Reaction with carbon dioxide
When carbon dioxide gas is bubbled through aqueous solutions of the caustic alkalis, the carbonates are formed.
2NaOH(aq) +CO2(g) → Na2CO3(aq) + H2O(l). With excess of the gas, the hydrogencarbonates are formed.Na2CO3(aq) + H2O(l) + CO2(g) → 2NaHCO3(aq)
Reaction with chlorine
Chlorine reacts with excess of cold dilute caustic alkalis to form the hypochlorite, (NaClO or KClO). 2NaOH(aq) + Cl2(g) → NaCl(aq) + NaClO(aq) + H2O(l).2KOH(aq) + Cl2(g) → KCl(aq) + KClO(aq) + H2O(l)
If excess chlorine is bubbled through hot concentrated solutions of caustic alkalis, the chlorates are formed, (NaClO3 or KClO3).
6NaOH(aq) + 3Cl2(g) → 5NaCl(aq) + NaClO3(aq) + 3H2O(l).6KOH(aq) + 3Cl2(g) → 5KCl(aq) + KClO3(aq) + 3H2O(l)
Applications of acid-base neutralization in everyday life
Applications of acid-base neutralization in everyday life
Acid-base neutralization has many applications in everyday life. The following are some of these applications:
Indigestion and pain relief
The dilute hydrochloric acid produced in your stomach is used for digestion and killing bacteria that might have been swallowed together with food or taken with water. However, excess acid causes indigestion, which can be painful. To ease the pain, we take an anti-acid treatment. Anti-acids are a broad group of compounds with no toxic effects on the body. They are used to neutralize the effects of acid indigestion.
Some of these anti-acids such as milk of magnesia [insoluble magnesium hydroxide, Mg(OH)2] help to neutralize and hence counteract the excess acid in the stomach. This treatment, therefore, prevents indigestion and pains. The neutralization reaction equation is:
Mg(OH)2(aq) + 2HCl(aq)→ MgCl2(aq) + 2H2O(l)
Other anti-acids such as “Alka-Seltzer” contain soluble materials, including sodium hydrogencarbonate. These tablets also contain some citric acid (a solid acid). On adding water, the acid and some of the sodium hydrogencarbonate react, producing carbon dioxide gas. This helps to spread and dissolve the other less soluble material. When taken, more sodium hydrogencarbonate neutralizes the excess hydrochloric acid in the stomach, thus easing digestion.
Some anti-acid tablets also contain painkiller to relieve pain. “Soluble aspirin” tablets dissolve and work in a similar way to “Alker-Seltzer” tablets. Vitamin C (ascorbic acid) can be added to the tablets. Note that it is important to add water to start the action of the acid.
Descaling kettles
The limescale (CaCO3) is formed inside boilers, kettles and water heaters when hard water is boiled. The limescale can be removed by treatment with an acid that is strong enough to react with CaCO3, but not strong enough to damage the metal. Vinegar can be used to discale kettles. Commercial “discalers” use other acid solutions such as methanoic acid
Prevention of tooth decay
Food remnants sticking onto teeth (plaque), after eating especially sugary food is acted upon by bacteria in your mouth. The pH of a sugar solution is 7. However, bacteria in your mouth break down the sugar in plaque to form acids, for example lactic acid. These acids lower the pH. Tooth decay begins when the pH falls below 5.8. The acid attacks the tooth enamel.
To help prevent tooth decay many types of toothpaste contain basic substances to neutralize the acids produced by these bacteria in your mouth. The pH of these basic substances is alkaline (higher than 7). The pH of saliva is slightly alkaline (pH 7.4), so it can also help to counteract the acid, particularly after a meal. After eating a sweat, for example, it takes about 15 minutes for saliva to raise the pH above 5.8, and stop further decay.
Soil treatment
Most plants grow best when pH of the soil is close to 7. They prefer the pH of between 6.5 and 7.0. If the soil pH is below 6.0, the soil is too acidic. Above the pH of 8.0, the soil is too alkaline. If the soil is too acidic or too alkaline, the plants grow poorly or not at all.
Chemicals can be added to the soil to adjust its pH. Most often, if the soil is too acidic, it is usually treated by liming. In this context, liming means addition of quicklime (calcium oxide), slaked lime (calcium hydroxide) or powdered chalk or limestone (calcium carbonate) to an acidic soil. These compounds (bases) have the effect of neutralizing the acidity of the soil.
If the soil is too alkaline, acids such as sulphuric acid, nitric acid or hydrochloric acid may be added to the soil to neutralize excessive alkalinity. However, these compounds are very expensive and hence uneconomical to apply on large-scale basis.
Insect stings treatment
When a bee stings someone, it injects an acid liquid into the skin. The bee sting, which is acidic in nature, can be neutralized by rubbing on calamine solution, which contains zinc carbonate or baking soda, which is sodium hydrogencarbonate. These compounds are basic in nature and so have the effect of neutralizing the acid in the sting.
Wasp stings are alkaline in nature, and can be neutralized with vinegar, which contains ethanoic acid. Ant and nettle stings contain methanoic acid. These may be neutralized by rubbing an extract squeezed from crushed onion leaves (which contain basic compounds) on the affected skin. The acid in the sting can also be neutralized by applying weak alkalis such as ammonia solution, ash extract, baking powder, etc.
Factory wastes treatment
Liquid wastes from factories often contain acid. If it reaches a river, lake or ocean, the acid will kill fish and other aquatic life. This can be prevented by adding slaked lime (calcium hydroxide) to the waste, to neutralize the acid before being dumped into water bodies.
Indicators
An Indicator from Locally Available Materials
Explain an indicator from locally available materials
Certain coloured substances (many extracted from plants) have been found to change colour if added to an acid or alkaline solution. The colour change is reversed if the acid or alkali is neutralized. Substances that behave like this are known as indicators.
Coloured extracts can be made from red cabbage or blackberries, but probably the most used indicator is litmus. This is extracted from lichens.
Litmus is purple in a neutral solution. When added to an acid solution, it turns red. Changing this red colour of litmus needs a chemical reaction. The molecules of the indicator are usually changed in the presence of the acid. Substances with the opposite chemical effect to acids are needed to reverse the change, and these are called alkalis. They turn litmus solution to blue. Litmus can also be used in paper form, in which case it is called litmus paper. Here it comes in the blue and red forms. Litmus is a single chemical compound. It gives a single colour change.
Litmus is not the only single indicator that chemists find useful. Others that are used frequently are phenolphthalein and methyl orange. These indicators give different colour changes when in acidic and alkaline solutions (see table 3.2).Another commonly used indicator is the universal indicator (or full-range indicator). This is made from a mixture of dyes. Such an indicator is useful because it gives a range of colours (“spectrum”) depending on the strength of the acid or alkali added (see table 3.3)
With a universal indicator, different acids produce a range of different colours. Indeed, solutions of the same acid with different concentrations (pH) give different colours.
The more acidic solutions (for example battery acid) turn the universal indicator bright red. A less acidic solution (for example vinegar) will only turn it orange-yellow. There are also colour differences produced with different alkali solutions. The most alkaline solutions give a violet colour while the less alkaline solutions give a blue colour.
We learned that many indicators are extracted from plants. Flowers and leaves of different plants have different colours. These plant organs may be used to prepare indicators locally.
The Acidity and Alkalinity of Substance Using Indicators
Test the acidity and alkalinity of substance using indicators
The Strengths of Acids and Bases
There is a big difference between the strength of an acid or base and its concentration. An acid or alkaline solution is said to be concentrated if it contains a large amount of it in a small amount of water. A dilute acid or base (alkali) has a small amount of it in a lot of water. The concentration of an acid or base tells us how much of it is dissolved in a certain volume of solution. The concentration is normally expressed in grams per litre (g dm-3) or moles per litre (mol dm-3).
The strength of an acid or alkali expresses its dissociation in water. Strong acids or alkalis will dissociate completely in water to form ions. Examples of strong acids are sulphuric acid, hydrochloric acid, nitric acid and phosphoric acid. Weak acids include ethanoic acid, carbonic acid and methanoic acid. Examples of strong alkalis include potassium hydroxide, sodium hydroxide, calcium hydroxide and ammonium hydroxide. Weak bases include ammonia solution and sodium hydrogencarbonate.
A strong acid or alkali forms many ions in water. The number of hydrogen ions, H+, formed when it dissociates in water, determines the strength of an acid. The strength of an alkali depends on the number of hydroxyl ions, OH-, formed when it dissociates in water. Strong acids and alkalis will form many H+ and OH- ions respectively. Weak acids or bases will form very few of the respective ions.
Likewise, the term weak acid or base should not be confused with the term dilute acid or base. A weak acid dissociates in water only very slightly to form very few protons, H+. A weak alkali also dissociates very slightly to form very few hydroxyl ions, OH-.
The Concept of an Indicator
Describe the concept of an indicator
You have seen that single indicators change their colours only once when put in different acid and alkaline solutions. The single indicators most commonly used include litmus, phenolphthalein and methyl orange.
On the other hand, universal indicators show a range of colour changes depending on the strength of an acid or base.
Single indicators can only tell us whether a certain solution is an acid or an alkali. These types of indicators cannot be used to compare two acids or two alkalis with different strengths. Litmus paper, for example, cannot be used to compare the strengths of sulphuric acid and ethanoic acid. Both acids will change the blue litmus paper to red. Likewise, you cannot compare the strengths of aqueous ammonia solution (NH4OH) and sodium hydroxide by just using a litmus paper. They will both turn to red litmus paper to blue.
A universal indicator can be used to measure strengths of different acids and alkalis. This indicator is a mixture of simple indicators. Instead of changing colour just once, it changes colour a number of times depending on the degree of acidity or alkalinity of the substances tested.
The pH scale is a convenient means of expressing the acidity and alkalinity in liquids. The pH scale is a numerical scale used to indicate the relative strengths of acidic or basic solutions in terms of relative amount of hydrogen ions (protons) or hydroxyl ions in solutions. The scale ranges from 0 to 14.
Acidic solutions will have pH values less than 7.0 and alkaline solutions will have pH values greater then 7.0. All neutral liquids e.g. pure water have pH of 7.0. Table 3.3 shows the pH and strengths of acidic and alkaline solutions and the associated indicator colour changes.
Colours of the universal indicator in different acidic and alkaline solutions
| pH range | Colour | Strength |
| 1, 2, 3 | Red | Strongly acidic |
| 4 | Orange | |
| 5, 6 | Yellow | Weakly acidic |
| 7 | Green | Neutral |
| 8, 9 | Blue Indigo | Weakly alkaline |
| 10, 11, 12, 13, 14 | Purple/violet | Strongly alkaline |
Remember that there is no clear dividing line between the pH ranges as apparently shown in the above table. This means that you may have substances with, for example, pH 1.2, 1.5, 3.5, 4.4, 5.6, 8.4, etc. The table just tries to simplify the concept of acidity and alkalinity of acid and alkaline solutions.
Salts
The Natural Source of Salts in Daily Life
Investigate the natural source of salts in daily life
A salt is a substance formed when some or all of the hydrogen atoms of an acid are replaced by a metal or ammonium ion. A salt, therefore, may be defined as a compound in which the replaceable hydrogen of an acid has been wholly or partially replaced by a metal.
In sodium chloride (NaCl), for example, the hydrogen atom of hydrochloric acid (HCl) has been wholly replaced by an atom of sodium. In magnesium sulphate (MgSO4) and sodium sulphate (Na2SO4), both hydrogen atoms of sulphuric acid (H2SO4) have been replaced by one atom of magnesium and two atoms of sodium respectively. In sodium hydrogen sulphate (NaHSO4), only one out of two hydrogen atoms has been replaced by an atom of sodium. This type of a salt is called an acid salt, because it still contains a replaceable hydrogen atom.
Many chemical compounds may be classified as salts. The salt most familiar to every body is table salt (sodium chloride). Baking soda is the salt, sodium bicarbonate (NaHCO3). Magnesium sulphate (also called Epsom salt) is often found in the home.
In general, salts are ionic impounds that are composed of metal and non metal ions. For example, sodium chloride is is composed of metallic sodium ions (Na+) and non-metallic chloride ions (Cl-). Some salts are made of metallic and non-metallic radicals e.g ammonium nitrate (NH4NO3) is composed of ammonium radical (NH4+) and nitrate radical (NO3-).
There is a wide range of types and natural sources of salts. Common salt is mined from underground deposits. The salt obtained from such a source contains sodium chloride mixed with rock impurities.
The other source of sodium chloride is seawater. The salty taste of seawater is due to the presence of salts such as sodium chloride and magnesium bromide. However, there are many different types of salts present in seawater, though in small proportions, as shown in the table below (table 3.5)
| Salt | Formula | Percentage composition |
| Sodium chloride | NaCl | 2.72 |
| Magnesium chloride | MgCl2 | 0.38 |
| Magnesium sulphate | MgSO4 | 0.17 |
| Calcium sulphate | CaSO4 | 0.13 |
| Potassium chloride | KCl | 0.09 |
| Calcium chloride | CaCO3 | 0.01 |
| Magnesium bromide | MgBr2 | 0.01 |
Sodium nitrate (Chile saltpetre), NaNO3 and calcium carbonate, CaCO3 are found in underground deposits. Calcium carbonate occurs naturally as marble, limestone or chalk in the ground from which it can be mined mechanically. What other natural sources of salts do you know?
Types of Salts
Salts may be classified according to their mode of formation. The following are types of salts grouped according to their mode of formation:
Normal salt:- This is a salt formed when all of the replaceable hydrogen atoms of an acid have been replaced by a metal atom e.g. sodium chloride is a normal salt because all hydrogen atoms are replaced from an acid during its formation.
2Na(s) + 2HCl(aq)→ 2NaCl(aq) + H2(g)
Other normal salts include magnesium chloride (MgCl2), potassium chloride (KCl), copper (II) sulphate (CuSO4), sodium sulphate (Na2SO4), sodium carbonate (Na2CO3), trisodium phosphate (Na3PO4), etc.
Acid salt:- An acid salt is a salt formed when part of the replaceable hydrogen atoms of an acid are displaced by a metal e.g., sodium bisulphate (NaHSO4) is an acid salt.
H2SO4(aq) + NaOH(aq)→ NaHSO4(aq) + H2O(l)
Other examples of acid salts include sodium hydrogensulphate, (NaHSO4), sodium hydrogensulphide, (NaHS) and sodium hydrogencarbonate (NaHCO3). Since acid salts contain hydrogen ions, they exhibit some acidic properties. Hence, they behave like acids, for example:
- they react with bases to form salts and water only. NaHSO4(aq) + NaOH(aq)→ Na2SO4(aq) + H2O(l)
- they react with carbonates to yield carbon dioxide.2NaHSO4(aq) + Na2CO3(aq)→ 2Na2SO4(aq) + H2O(l) + CO2(g)
Basic salt:- A basic salt is formed by the action of an acid with higher proportions of the base, than is necessary for the formation of a normal salt.
Examples of basic salts are:
- Basic copper carbonate, CuCO3.Cu(OH)2
- Basic lead carbonate (white lead), PbCO3.Pb(OH)2
- Basic magnesium chloride, MgCl2.Mg(OH)2
- Basic zinc chloride, ZnCl2.Zn(OH)2
A basic salt may also be formed by the partial replacement of the hydroxyl groups of a diacidic or triacidic base by an acid radical.
Pb(OH)2(s) [lead hydroxide] + HNO3(aq) [nitric acid] → Pb(OH)NO3(s) [basic lead nitrate]+ H2O(l)[water]
Basic salts are usually insoluble in water. Such salts are formed by the close association of two simple salts, when crystallized from a solution of a mixture of the two.
The Solubility of Different Salts in the Laboratory
Analyse the solubility of different salts in the laboratory
Some salts are more soluble in water than others are. However, other salts are insoluble in water. The knowledge of solubility of different salts in water is very important because it can help us prepare different salts in the laboratory by such methods as precipitation, direct combination (synthesis), crystallization and so forth.
As regards to solubilities, salts can be classified into two groups: salts which are soluble in water (soluble salts) and salts which do not dissolve in water (insoluble salts). Table 3.6 summarizes the solubility of different salts in water.
The patterns of solubility for various types of salts
| Soluble salts | Insoluble salts |
| 1. All sodium, potassium and ammonium salts. | silver, mercury(I) and lead chlorides barium, lead (II) and calcium sulphates but other common carbonates are insoluble.but other common hydroxides are insoluble. |
| 2. All nitrates of metals | |
| 3. All chlorides except .……………........ | |
| 4. All sulphates except………………….. | |
| 5. Sodium, potassium, and ammonium carbonates…………………………... hydroxides………………………….…… | |
| 6. Sodium, potassium and ammonium |
Salts in the Laboratory
Prepare salts in the laboratory
Several methods are available for the preparation of salts. The solubilities of the prepared salts determine their methods of preparation. Hence, in the choice of a method of preparation of a particular salt, one has to be acquainted with its solubility properties.
Soluble salts are usually prepared by methods which involve crystallization. In this method, as the name suggests, resultant salts are in the form of crystals.
Insoluble salts are usually prepared by methods which involve precipitation. These methods are sometimes referred to as double decomposition. To precipitate an insoluble salt, you must mix a solution that contains its positive ions with the one that contains its negative ions.
Salts may also be prepared by direct combination (or synthesis). For example, magnesium chloride may be prepared in the laboratory by heating magnesium in a stream of chlorine.
Mg(s) + Cl2(g) → MgCl2(s)
Preparation of soluble salts
Soluble salts may be prepared by any of the following methods:
- Reaction between an acid and an alkali: In this method, a dilute acid is added to an alkali in the appropriate volume ratio. The reaction between an acid and an alkali is termed as neutralization. For example, sodium chloride may be prepared by the following neutralization reaction:NaOH(aq)+ HCl(aq)→ NaCl(aq) + H2O(l). Both reactants are soluble, and no gas is given off during the reaction. So, it is difficult to know when the reaction is over. In this case, you have to use an indicator. A universal indicator or litmus could be used, but even better is phenolphthalein. This is pink in alkaline solution, but colourless in neutral or acidic solutions.
- Reaction of a metal with an acid: This is another general method for preparing salts. For example, zinc sulphate can be made by reacting dilute sulphuric acid with zinc:Zn(s) + H2SO4(aq)→ ZnSO4(aq) + H2(g). However, this method is not suitable for all metals or all acids. It is good for preparing salts of fairly reactive metals such as magnesium, aluminium, zinc and iron. However, the reactions of highly reactive metals like sodium, potassium and calcium with acids are very violent and dangerous. The reaction with lead is too slow. Copper, silver and gold do not react at all.
- Reaction of a metal oxide with an acid. Metal oxides, as you studied early, react with dilute acids to produce salts. Copper oxide is an insoluble base. Although copper will not react with dilute sulphuric acid, copper (II) oxide will. The salt that forms is copper (II) sulphate.CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(l)
- Reaction of a metal carbonate with an acid. The reaction between metal carbonates and dilute acids are accompanied with evolution of carbon dioxide gas. The evolution of a gas can be used to indicate when the reaction is over. An example of such reactions is the reaction between calcium carbonate and dilute hydrochloric acid. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
General methods of preparing soluble salts
The above methods for preparing soluble salts are specific for each method mentioned. Generally, soluble salts may be prepared by two broad methods.
Method 1: This route is essentially the same whether starting with a solid metal, a solid base (oxide) or a solid carbonate. The route can be divided into four stages:
- Stage 1: An excess (more than enough) of the solid is added to the acid and allowed to react. Using an excess of the solid makes sure that all the acid used up. If it is not used up at this stage, the acid would become more concentrated when the water is evaporated later (stage 3).
- Stage 2: The excess solid is filtered out after the reaction is completed.
- Stage 3: The filtrate is gently evaporated to concentrate the solution. This can be done on a heated water bath. Do not heat so strongly or “spitting” might take place.
- Stage 4: The concentrated solution is cooled down to let the crystals form. Filter off the crystals. Wash them with a little distilled water. Dry the crystals carefully between the filter papers.
Method 2: This method (titration method) involves the neutralization of an acid with an alkali (for example sodium hydroxide) or a soluble carbonate (for example sodium carbonate). Since both the reactants and the products are colourless, an indicator is used to find the neutralization point or end point (when all the acid has just been neutralized). Once the end point is reached, the resulting salt solution is evaporated and cooled to form crystals as described in method 1.
General methods for preparing insoluble salts
Some salts are insoluble in water (for example silver chloride and barium sulphate – see table 3.6). Such salts are generally prepared by ionic precipitation.Precipitation is the sudden formation of a solid either: when two solutions are mixed; or when a gas is bubbled into a solution.
For example, barium sulphate can be prepared by adding a solution of a soluble sulphate (for example sodium sulphate) to a solution of a soluble barium salt (for example barium chloride). The insoluble barium sulphate is formed immediately. This solid falls to the bottom of the container as a precipitate (figure 3.2). The precipitate can be filtered off. It is then washed with distilled water and dried in a warm oven. The equation for the reaction is:
BaCl2(aq) + Na2SO4(aq)→ BaSO4(s) + 2NaCl(aq)
This shows how important the state symbols can be - it is only through state symbols that we can tell this equation shows a precipitation.
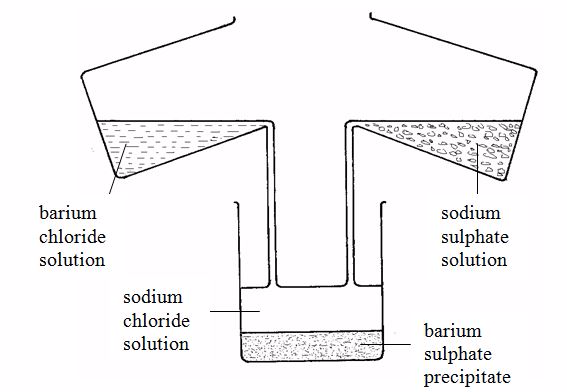
Barium sulphate could also be made from barium nitrate and sodium sulphate, for example, since these salts are both soluble. As long as barium and sulphate ions are present, barium sulphate will be precipitated.
Ba2+(aq) + SO42-(aq) →BaSO4(s)
Precipitation reactions are often used in a qualitative analysis to identify salts such as chlorides, iodides and sulphates.
Preparation of salts by direct combination (synthesis)
Some soluble and insoluble salts can be made directly by reacting two elements together. This is called combination (or synthesis). This type of reaction is mainly possible for metal chlorides, bromides and iodides. For instance, if a piece of burning sodium is lowered into a gas jar of chlorine, the two react violently to produce a white powder of sodium chloride:
2Na(s) + Cl2(g) → 2NaCl(s)
Other chlorides can also be prepared by combination, for example, iron (III) chloride and aluminium chloride can be made by heating iron and aluminium metals in stream of chlorine:
2Fe(s) + 3Cl2(g) → 2FeCl3(s)
2Al(s) + 3Cl2(g) → 2AlCl3(s)
The reaction between ammonia gas and hydrogen chloride gas to produce ammonium chloride is also a synthesis reaction.
NH3(g) + HCl(g) → NH4Cl(s)
Direct combination reactions do not produce crystals of the salt, but only a powder.
The Effects of Heat on Salts
Examine the effects of heat on salts
When different salts are heated, they behave in different manners. The crystals of some salts contain water of crystallization. When these hydrated salts are heated, their water of crystallization is driven off as steam. The crystals then lose their shape and become a powder. The following are few examples of hydrated salts:
| Salt formula | Chemical name |
| CuSO4.5H2O | Copper (II) sulphate five water |
| Na2CO3.I0H2O | Sodium carbonate ten water |
| MgCl2.6H2O | Magnesium chloride six water |
| FeCl3.6H2O | Iron (III) chloride six water |
| FeSO4.7H2O | Iron (II) sulphate seven water |
| CoCl2.6H2O | Cobalt (II) chloride six water |
| MgSO4.7H2O | Magnesium sulphate seven water |
| CaSO4.2H2O | Calcium sulphate two water |
Sulphates
Sulphates of potassium, sodium, calcium, lithium and magnesium are stable to heat and do not decompose when heated. Other sulphates decompose to give the oxide and sulphur trioxide gas except iron (III) sulphate which decomposes to give sulphur dioxide and sulphur trioxide.
Copper (II) sulphate five water crystals are blue in colour, but when heated, they are dehydrated to form a white powder:
CuSO4.5H2O(s)hydrated (blue)→ CuSO4(s)anhydrous (white) + 5H2O(g)
Crystals that have lost their water of crystallization are called anhydrous. If water is added back to the anhydrous copper (II) sulphate powder, the powder turns into blue crystals again and heat is evolved. This can be used as a qualitative test for water.If the white, anhydrous powder is further heated strongly, it decomposes to black copper (II) oxide:
CuSO4(s)white→ CuO(s)black+ SO3(g)
Hydrated iron (II) sulphate is green in colour. When heated, it loses all its water of crystallization and changes colour from green to white:
FeSO4.7H2O(s)green→ FeSO4(s)white + 7H2O(g)
When heated even more strongly, the white powder decomposes to form a black oxide:
2FeSO4(s)white→ Fe2O3(s)black+ SO2(g) + SO3(g)
Iron (III) sulphate decomposes on heating to form slightly different products:
Fe2(SO4)3(s) → Fe2O3 + 3SO3(g)
Chlorides
The chlorides of most metals are hydrated except those of potassium, lead, mercury and silver. Hydrated chlorides do not usually give the anhydrous salt when heated. Instead, a chemical change termed as hydrolysis normally occurs. The reaction is accompanied by the evolution of steam and hydrogen chloride gas, and the formation of the basic chloride or oxide. When, for example, hydrated magnesium chloride is heated, its basic chloride is formed:
MgCl2.6H2O(s) → Mg (OH)Cl(s) + HCl(g) + 5H2O(g)
The same case applies when hydrated calcium chloride is heated. However, when hydrated aluminum chloride is heated, it does not produce the anhydrous salt. Instead, the oxide is formed thus:
2AlCl3.6H2O(s) → Al2O3(s) + 6HCl(g) + 3H2O(g)
Ammonium chloride sublimes when heated. The reaction is reversible and the products may recombine on cooling to form the salt back.
NH4Cl=⇔NH3(g) + HCl(g)
Carbonates and hydrogencarbonates
The carbonates of potassium and sodium are very stable to heat. They do not decompose even when heated to very high temperatures. All other carbonates decompose when heated to give the oxide and carbon dioxide:
CaCO3(s) ⇔CaO(s) + CO2(g)
CuCO3(s) → CuO(s) + CO2(g)
However, there are very few and exceptional carbonates that do not behave like this. Ammonium carbonate, for example, decomposes readily when heated to give ammonia gas, water vapour and carbon dioxide gas:
(NH4)2CO3(s) → 2NH3(g) + H2O(g) + CO2(g)
All hydrogencarbonates decompose on heating to give the carbonates, water vapour and carbon dioxide:
2NaHCO3(s) → Na2CO3(s) + H2O(g) CO2(g)
Nitrates
When heated, potassium and sodium nitrates decompose to give the nitrite and oxygen:
2KNO3(s) → 2KNO2(s) + O2(g)
2NaNO3(s) → 2NaNO2(s) + O2(g)
The nitrates of common heavy metals (such as Pb, Al, Ca, Mg, Zn and Cu) decompose on heating to give the oxide, nitrogen dioxide and oxygen:
2Pb(NO3)2(s) → 2PbO(s) + 4NO2 (g) + O2 (g)
2Ca(NO3)2(s) → 2CaO(s) + 4NO2 (g) + O2 (g)
The nitrates of silver and mercury are completely decomposed to the metal, nitrogen dioxide and oxygen:
2AgNO3(s) → 2Ag (g) + 2NO2 (g) + O2 (g)
Hg(NO3)2(s) → Hg(l) + 2NO2(g) + O2(g)
Ammonium nitrate is decomposed by heat into dinitrogen oxide and water:
NH4NO3(s) → N2O (g) + 2H2O (l)
Hydroxides
Potassium and sodium hydroxides are very stable to heat. They do not decompose even when heated strongly. All other hydroxides decompose to give the oxide and water vapour, e.g.:
Ca(OH)2(s) → CaO(s) + H2O(g)
DELIQUESCENCE, EFFLORESCENCE AND HYGROSCOPY
Deliquescence
Deliquescence is the absorbing of moisture from the atmosphere by a solid to form a solution.If calcium chloride (CaCl2) is exposed to air, it absorbs water vapour from the atmosphere and eventually dissolves. Its tendency to absorb water vapour explains why it is used as a drying agent for gases (not ammonia, because it combines with the gas).
Solid sodium hydroxide is also deliquescent. On exposure to air, pellets of sodium hydroxide quickly become shiny and then sticky as they absorb water vapour from the atmosphere. Eventually the sodium hydroxide pellets absorb moisture from the atmosphere so much that they dissolve to form a solution of sodium hydroxide.
Copper (II) nitrate and zinc chloride are the other deliquescent salts. Pure table salt (NaCl) is not deliquescent. However, if the salt is directly obtained from the sea, it is deliquescent. The salt from the sea contains magnesium chloride as one as the impurities. It is this magnesium chloride salt that deliquesces and not sodium chloride.
Hygroscopy
Some substances tend to absorb water vapour from the air but do not change their physical states. Copper (II) oxide and calcium oxide are both hygroscopic solids because they can absorb moisture from the atmosphere and yet retain their solid states. Because of this behaviour, calcium oxide is used as a drying agent, which absorbs moisture from gases prepared in the laboratory.
Concentrated sulphuric acid is a hygroscopic liquid. When exposed to air, the acid absorbs water vapour from the atmosphere diluting itself to absorb 3 times its original volume.
Therefore, hygroscopy may be defined as the tendency of a substance to absorb water vapour from the atmosphere without changing its physical states.The word hygroscopy is a general term applied to all substances that absorb water vapour from the air. Any substance that can take up moisture from the atmosphere is said to be hygroscopic in nature.
Efflorescence
Efflorescence is the tendency of a hydrated substance to lose the water of crystallization to the atmosphere. Some salt crystals give out some or all of their water of crystallization to the atmosphere when exposed to air. Such substances are said to be efflorescent and the process of water loss is known as efflorescence. Sodium carbonate ten water (washing soda) is a good example of an efflorescent substance. If washing soda crystals are exposed to open air at room temperature, they lose some of the water of crystallization. The solid loses nine of its ten molecules of water of crystallization to the air. One molecule of water, which remains fixed, can be removed only by strong heating.
Na2CO3.10H2O(s)crystals → Na2CO3.H2O(s)powder + 9H2O(g)
The crystal lattice is broken down as the salt loses its after of crystallization. Thus, transparent crystals of hydrated sodium carbonates become white and powdery on the surface.
Another efflorescent compound is Glauber’s salt, sodium sulphate ten water (Na2SO4.10H2O). On exposure to air, it loses the whole of its water of crystallization to the air.
Na2SO4.10H2O(s) → Na2SO4(s) + 10H2O(g)
Iron (II) sulphate seven water (FeSO4.7H2O) is also efflorescent.
The Uses of Different Types of Salts in Everyday Life
Explain the uses of different types of salts in everyday life
There is a wide range of salts. A great number of them play an important role in our everyday life. The following are the uses of some salts:
Sodium chloride (common salt)
Sodium chloride often called common salt or table salt, is essential for life and is an important raw material for industries. At home, it is used for cooking, that is, flavouring different foods. Biologically, it has a number of functions: it is involved in muscle contraction; it enables the conduction of nerve impulses in the nervous system; it regulates osmosis (the passage of solvent molecules through membranes); and it is converted into the hydrochloric acid that aids digestions in the stomach.
Some industrial uses of sodium chloride include curing bacon, flavouring foods, and in the manufacture of margarine, butter and cheese. It is also used to tan leather in the leather industry. Rock salt is used as a fertilizer for sugar beet, and is spread on roads to melt the ice during winter. The salt is the starting point for many important chemicals, for example, the electrolysis of brine (concentrated solution of sodium chloride) gives sodium hydroxide, chlorine and hydrogen. What other uses of sodium chloride do you know? Mention them.
Calcium carbonate (marble, limestone, chalk)
Calcium carbonate finds a wide range of uses:
- An important use of calcium carbonate is in the building industry. It is widely used in making cement, lime, mortar and making steel from iron.
- Powdered limestone is used as a liming material to neutralize soil acidity. When used in this way, it is termed as agricultural lime. When added in the soil, agricultural lime acts as a calcium source for plants as well as increasing the pH and water retaining capacity of acidic soils.
- It is also used in making paint, plastic, rubber, ceramic and glass; and in oil refining, and iron ore purification.
- Calcium carbonate is the most preferred mineral in the paper industry. It helps in the production of the best quality papers.
- Since calcium is essential for healthy bones and teeth, it is used as a dietary calcium supplement.
- Limestone can also be well shaped, painted, and then used as decorative stones.
Ammonium salts
Most ammonium salts such as (NH4)3PO4, NH4Cl, NH4NO3, (NH4)2SO4, CAN, urea, etc are used as nitrogenous fertilizers which are applied to the soil to improve soil fertility and hence enhance plant growth and production. Millions of tonnes of fertilizers are produced every year. Without these chemicals, world food production would probably be halved.
Calcium sulphate (anhydride, CaSO4; gypsum, CaSO4.H2O)
Gypsum is chiefly used for the manufacture of Plaster of Paris (P.O.P). The Plaster of Paris is used for making casts for statuary ( the expansion during setting ensures a fine impression), in surgery to maintain joints in a fixed position and in cement and wall plasters.
Among the many other uses of calcium sulphate are as a pigment in white paints, as a soil conditioner, in Portland cement, as a sizer, filler, and coating agent in papers, in the manufacture of sulphuric acid and sulphur, in the metallurgy of zinc ores and as a drying agent in many laboratory and commercial processes.
Sodium carbonate (washing soda)
Sodium carbonate is widely used as one of the raw materials for making glass. Glass is made by heating a mixture of limestone, sand, sodium carbonate, and recycled glass in a furnace.The cosmetic industry uses it for manufacturing soap. The chemical industry uses it as a precursor to numerous sodium-containing reagents
It is also important in photography and in the textile industry. In addition to these industrial applications, sodium carbonate is used in medicine as an anti-acid
Sodium carbonate has various environmental applications. Large quantities of the carbonate are used in sewage treatment; in water softening as washing soda crystals, Na2CO3.10H2O, and in desulphurisation of flue gas.
Magnesium sulphate (Epsom salt)
Magnesium sulphate is mainly employed for making health salts (laxative, mild purgative). The health salt is used as a medicine (laxative) which aids to empty the bowels following constipation or other health problems.
Copper (II) sulphate
Copper (II) sulphate is used in fungicides, which are sprayed on crops, especially vines and potatoes, to kill moulds which would injure plants and hence curtail crop yield. It is also used in the manufacture of certain green pigments which are used for painting.
Calcium phosphate
It is largely used in making phosphoric acid and fertilizers. Calcium phosphate is used in baking. It is also used in cheese products.






Notes are too shallow
ReplyDelete