Join Our Groups
TOPIC 2: HARDNESS OF WATER
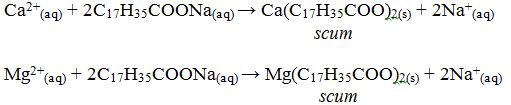
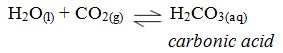
The Concept of Hardness of Water
The Concept of Hardness of Water
Explain the concept of hardness of water
As water flows over the land, it dissolves many mineral substances. The dissolved minerals are deposited together with water in rivers, lakes and oceans. Water is said to be hard if it contains some specific type of dissolved minerals. It is important to note that not all dissolved salts make water hard.
As you learned early, water is treated in water purification plants before being piped to your home. The treatment removes only the insoluble particles and kills bacteria. So the water still is not pure. It contains natural compounds dissolved from rocks and soil. It may also contain traces of chemicals dumped from homes, farms and factories.
Water obtained from an area where the rocks contains chalk, limestone, dolomite or gypsum, contains dissolved calcium and magnesium sulphates and hydrogen carbonates. These salts make the water hard.
One can distinguish between hard and soft water when washing with soap. Hard water does not form lather easily. Instead, it forms a precipitate or scum. It requires much soap to react with all the dissolved minerals before enough lather is formed. Therefore, hard water wastes soap during washing.
When soap is used with hard water a “scum” forms on the surface. This is a result of a precipitation reaction between calcium and/or magnesium ions and soap. Soaps are the sodium or potassium salts of long-chain organic acids. Soaps are made from animal fats by treatment with alkali (NaOH or KOH). Ordinary washing soap is a compound of stearic acid, C17H35COOH. The nature of such soaps is the salt, sodium stearate, C17H35COONa+. Sodium stearate is soluble in water but calcium stearate is not.
When soap is mixed with hard water, the calcium or magnesium salts in the hard water react with soap and precipitates as scum. The nature of scum is either calcium stearate or magnesium stearate:
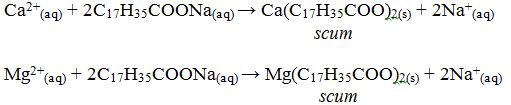
Soap will not form any lather with water until all the calcium and magnesium ions have been precipitated. Hard water, therefore, wastes soap. This means that more soap may be used for an efficient washing. The amount of soap needed to just produce froth can be used to estimate the hardness of water.
The problem of scum formation only occurs with soaps. Soapless detergents do not produce scum. The trade names for some soapy detergents sold in Tanzania include Komoa, Kuku, Taifa, Mbuni, Mshindi, Changu, Jamaa and several other bar soaps. The trade names for some soapless detergents include Omo, Foma, Tesa, Toss, Dynamo, Swan, etc.
Causes of Permanent and Temporary Hardness in Water
State causes of permanent and temporary hardness in water
Water is generally said to be hard if it contains soluble salts of calcium and magnesium. The salts are calcium and magnesium sulphates and hydrogen carbonates. Hardness of water is caused by higher than usual levels of calcium (Ca2+) and magnesium (Mg2+) ions in water.
As rain water passes through the atmosphere, it dissolves carbondioxide to form a weak carbonic acid.
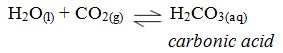
As this solution passes over and through rocks containing chalk, limestone or dolomite, the rainwater very slowly dissolves them:
H2CO3(aq) + CaCO3(s) → Ca(HCO3)2 (aq)
The calcium hydrogen carbonate formed is soluble in water and is responsible for the presence of calcium (Ca2+) ions in water.
Some of the rocks may contain gypsum (CaSO4.2H2O), an hydrite (CaSO4), Kieserite (MgSO4.H2O) or dolomite (CaCO3. MgCO3) which can dissolve to a limited extent in water. The presence of these dissolved substances also causes the water to be hard. These substances dissolve sparingly in water to form Ca2+ and Mg2+ ions which are responsible for water hardness as stated early.
Activity 1
Investigation of the causes of water hardness
Materials:
- Test tube rack-
- Five clean test tubes
- Measuring cylinder (100cm3)
- Calcium sulphate solution (1 mol dm-3)
- Soap solution
- Magnesium sulphate solution (1 mol dm-3)
- Sodium sulphate solution (1 mol dm-3)
- Potassium sulphate solution (1 mol dm-3)
- Distilled water
Procedure:
- Label five clean and dry test tubes as A, B, C, D and E. 2
- Add 10 cm3 of 1.0M calcium, magnesium, sodium and potassium sulphate solutions and distilled water in each of the test tubes respectively.
- Add 5 cm3 of soap in each test tube
- Shake the test tubes well and place them in a test tube rack
- Observe the amount of lather formed in each test tube, and if there is any precipitate (scum) formed.
Results:
Results of experiment showing minerals which cause water hardness
| Test tube | Salt present | Ions present in solution of salt | Lather or scum formed? | Water hard or soft? |
| A | calcium sulphate | Ca2+, SO42- | scum is formed | Hard |
| B | magnesium sulphate | Mg2+ , SO42- | scum is formed | hard |
| C | sodium sulphate | Na+, SO42- | lather is formed | soft |
| D | potassium sulphate | K+, SO42- | lather is formed | soft |
| E | distilled water | no ions | lather is formed | soft |
Interpretation of the results
From the result of experiment, we can conclude that scum is produced when either calcium or magnesium salt is present in water. So, high levels of calcium or magnesium ions in water are responsible for water hardness.
When the concentration of either of these minerals is over 150 milligrams per cubic decimeter (150 mg/dm3), water is considered to be hard. The upper limit allowed is 300 mg/dm3
Types of Hardness of Water
Types of Hardness of Water
Identify types of hardness of water
The hard water in some areas can be softened simply by boiling the water, but this is not true in all cases. This means that the hardness in water can be divided into two types – temporary and permanent hardness.
Temporary hardness
Temporary hardness in water is caused by dissolved calcium or magnesium hydrogencarbonates. The most important characteristic of temporarily hard water is that it can be softened by simply boiling. When the water is boiled, the soluble sodium hydrogencarbonate is decomposed to form the insoluble calcium carbonate.
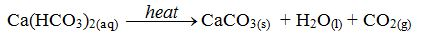
The decomposition causes the “furring” of kettles, hot water pipes and shower heads. This means that the inside of kettles, pipes and shower heads become coated with a layer of calcium carbonate (limescale) caused by the decomposition of the hydrogen carbonate according to the equation above.
In many supermarkets, it is possible to buy a limescale remover. This is often a solution of methanoic acid (formic acid). This weak acid is strong enough to react with limescale but not with the metal. The insoluble limescale (carbonate) is probably dissolved to a soluble compound, calcium methanoate that can be flushed away with water.
2COOH(aq) + CaCO3(s)(insoluble)→ Ca(HCOO)2(aq) + H2O(l) + CO2(g)calcium methanoate (soluble)
Permanent hardness
Permanent hardness in water is caused by soluble sulphates and chlorides of calcium and magnesium (CaSO4, MgSO4, CaCl2 and MgCl2). This type of hardness cannot be removed by boiling the water. This is because boiling does not decompose the chlorides of calcium or magnesium. Such water may only be softened by chemical treatment or ion exchange methods
The Difference between Soft and Hard Water
Differentiate soft from hard water
Activity 2
Distinction between temporarily and permanently hard water
Materials:
- Calcium carbonate
- Dilute hydrochloric acid
- 4 test tubes
- Test tube rack
- 1 litre of distilled water
- Calcium chloride
- Soap solution
- Beaker
- Heat source
Method:
- Prepare carbon dioxide gas in the laboratory by mixing calcium carbonate with hydrochloric acid in a gas generator.
- Bubble the gas through a suspension of calcium carbonate in water. Shake well as you bubble the gas until most of calcium carbonate has dissolved. Filter and divide the filtrate into two test tubes M and N.
- Prepare a 0.5M solution of calcium chloride and divide it in two test tubes P and Q.
- Prepare soap solution in a large beaker.
- Arrange the four test tubes in a rack
- Heat the solutions in test tubes M and P to boiling. Allow them to cool.7. Add 10cm3 of the soap solution to each of the four test tubes, M, N, P and Q. Shake well and allow to rest. Observe lathering and scum formation.
Note:
When calcium carbonate is reacted with dilute hydrochloric acid, carbon dioxide gas is produced.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
When carbon dioxide gas is bubbled through a suspension of calcium carbonate in water for a long time, the insoluble calcium carbonate dissolves to give the soluble bicarbonate, the presence of which makes the water hard.
CO2(g) + CaCO3(s) + H2O(l) → Ca(HCO3)2(aq)
The purpose of heating solutions in test tubes M and P was to try to remove water hardness. However, only the hardness in test tube M was merely removed by boiling because it contains the temporarily hard water.
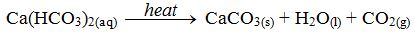
The hardness in test tube P could not be removed by just boiling because it contained the hard water. Calcium chloride cannot be decomposed by heat. So, no change is expected after heating.
Results:
- Scum was formed in test tubes N, P and Q but P and Q contained more scum than N.
- Lather was formed in test tube M only.
- Test tube M contained temporarily hard water and test tube P contained permanently hard water. The hardness in test tube M was removed by boiling while that in test tube P was not.
- Test tubes P and Q contained permanently hard water. The hardness in this water could not be removed by mere boiling.
Treatment and Purification of Hard Water
Process of Hard Water Treatment and Purification
Examine process of hard water treatment and purification
Because of the problem it causes, hard water is often softened for use in factories, industries and homes. That means removing the dissolved calcium and magnesium ions. Described below are the methods of treating and purifying hard water.
Boiling
Boiling removes temporary hardness in water, as you saw early. Boiling causes calcium carbonate to precipitate. The hydrogencarbonate in water are decomposed to carbonates, which are insoluble in water
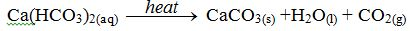
In this way, the calcium is removed, since the calcium carbonates being insoluble, takes no further part in the reaction. An insoluble calcium salt cannot cause hardness. However, this method uses a lot of fuel, which makes it expensive to do on a large scale.
Distillation
Distillation removes all impurities from water. This gets rid of both temporary and permanent hardness. In distillation, the water is boiled and the steam collected, cooled and condensed. Distilled water is pure and softest water. All the dissolved substances have been removed. Like boiling, it is an expensive option in terms of fuel used. But it is essential for some purposes, for example for laboratory experiments and for making drugs.
Addition of calcium hydroxide
Addition of calculated amounts of calcium hydroxide can remove temporary hardness. The quantity to be added should be properly calculated because excess would cause hardness on its own account. The amount of calcium hydroxide to be added is calculated based on knowledge of the hardness of water and the capacity of the reservoir (Clark’s method). The calcium hydroxide reacts with the hydrogen carbonates dissolved in water and precipitates as the insoluble calcium carbonates.
Ca(OH)2(s) slightly soluble + Ca(HCO3)2(aq) → 2CaCO3(s) + 2H2O(l) insoluble
Addition of sodium carbonate (washing soda)
Washing soda removes both temporary and permanent hardness by precipitating calcium carbonate. It reacts with calcium hydrogen-carbonate (which causes temporary hardness) to form sodium hydrogen-carbonate like this:
Na2CO3(aq)+ Ca(HCO3)2(aq)→ 2NaHCO3(aq))+ CaCO3(s)
It also reacts with calcium sulphate (which causes permanent hardness) to form sodium sulphate thus:Na2CO3(aq)+ CaSO4(aq)→ CaCO3(s)+ Na2SO4(aq)
These sodium salts are soluble, but do not cause the water to be hard. The calcium and magnesium ions are precipitated as insoluble calcium and magnesium carbonates. Ionically, the situation is like this:
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
Mg2+(aq)+ CO32-(aq)→ MgCO3(s)
Ion Exchange
Another method that removes both temporary and permanent hardness in water is the use of ion exchange resin. A typical ion exchanger is a container full of small beads. These beads are made of special plastic called ion exchange resin. The resin beads are porous and contain sodium ions. When hard water flows through the resin, the calcium and magnesium ions in the water are exchanged for the sodium ions and attach themselves to the resin. This process, therefore, removes calcium and magnesium ions from the water. They are replaced by sodium ions, which do not make the water hard.
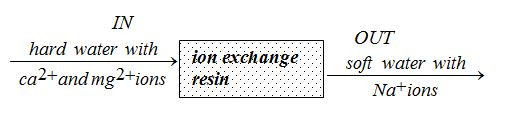
An ion exchange column removes ca2+ and mg2+ ions from the water and replaces them with Na+ ions
When all sodium ions have been removed from the resin, it is regenerated by pouring a concentrated solution of sodium chloride through it. The sodium ions remove the calcium and/or magnesium ions off the resin and the ion exchanger is ready for the use again. Other ions could also be used instead of sodium for the resin. But sodium chloride is normally used to supply the sodium ions because salt is cheap.
Use of softeners
Many modern washing powders now have softeners added to them. The softeners are often phosphates. The phosphates ions react with calcium ions to form calcium phosphate and remove the hardness. 3Ca2+(aq)+ 2PO43-(aq)→ Ca3(PO4)2(s)
The Importance of Hard Water Treatment and Purification
Describe the importance of hard water treatment and purification
The significance of water in daily life is well known to everyone. The water we obtain from natural sources is never pure. It contains dissolved minerals which render the water unfit for direct uses. The water from some sources contains calcium and magnesium compounds dissolved in it. These compounds are responsible for water hardness. To make the water fit for various uses, it is imperative to remove the hardness. The following points state why it is important to treat and purify hard water:
Hard water wastes soap. To get enough lather with hard water, it requires more soap than it does with soft water. So it is important to soften the water in order to save the soap and hence reduce the cost of washing. Laundry uses less soap and can be done at lower temperatures.
Treating and purifying hard water eliminates the possibility of forming limescale deposits in water boilers, kettles, washing machines, water heaters, shower heads and dish washers. The scale formed around the heating elements can cause the element to overheat and fail.
Treated and purified water leaves no scum on clothes during washing. Scum spoils the finishing of some fabrics. It forms nasty deposits (marks) on clothing that has been washed.
Softened water has the advantage of not blocking the water pipes. In industry, deposits of scales can block the pipes in boilers. This is a safety hazard as it could cause pressure to build up until there is an explosion. A similar coating can occur in hot water pipes at home and in central heating systems.
The Importance of Hard Water in Daily Life
State the importance of hard water in daily life
Hard water is not always disadvantageous. The following points explain the importance of hard water:
- The dissolved calcium and magnesium salts improve the taste of water. Distilled water is tasteless and quite unpleasant to drink. This is why water-processing plants add some salts in the distilled water to make it tasteful.
- Calcium dissolved in hard water is an essential mineral for growth of bones and teeth. It makes our teeth and bones hard, strong and resistant to shear and pressure.
- In some places, old lead pipes are used for water supply. Lead is very poisonous, and a little of it can dissolve in soft water. But the carbonate (CO32-) or sulphate (SO42-) ions present in hard water reacts with lead to form a coating of lead carbonate or lead sulphate that prevents lead from dissolving. This prevents lead poisoning.
- A coating of calcium carbonate inside pipes, boilers and radiators helps to prevent corrosion.
- In recent years, it has been suggested that drinking hard water helps to prevent heart diseases. 6. It has also been found that hard water is good for brewing beer.






I like the notes
ReplyDelete