Join Our Groups
TOPIC 1: OXYGEN
Oxygen exists in air to an extent of 21% by volume (or 23% by weight). It is the most abundant element on earth, accounting for ½ the total mass of the earth’s crust. Oxygen is mainly found in combined states as oxides, hydroxides, silicates, sulphates, carbonates, water, etc. Its ease of combination with other elements to form compounds shows that oxygen is a very reactive element.
Preparation and Properties of Oxygen
Oxygen can be prepared in the laboratory from either hydrogen peroxide solution or potassium chlorate salt.
A Sample of Oxygen Gas in the Laboratory
Prepare a sample of oxygen gas in the laboratory
(i) Laboratory preparation of oxygen from hydrogen peroxide solution
The most common method for the preparation of oxygen in the laboratory is by decomposition of hydrogen peroxide solution. The gas is prepared by catalysing the decomposition of hydrogen peroxide with manganese (IV) oxide. At room temperature hydrogen peroxide decomposes (breaks down) very slowly. It decomposes to water and oxygen.
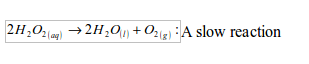
To speed up the decomposition process, and hence collect substantial amount of oxygen gas within a short time, black manganese (IV) oxide is added as a catalyst.
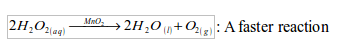
A catalyst is a substance that, although present in small quantities, will alter the rate of a chemical reaction but will remain chemically unchanged at the end of the reaction.
Preparation method
Hydrogen peroxide (20 vol.) is added drop by drop to manganese (IV) oxide, which catalyses the decomposition of the peroxide. Oxygen is collected over water as shown in figure bellow. The gas is collected by downward displacement of water because it is only slightly soluble in water.

Apparatus for laboratory preparation of oxygen from hydrogen peroxide solution
(ii) Laboratory preparation of oxygen from potassium chlorate
Oxygen can also be prepared by thermal decomposition of potassium chlorate. When this compound is heated, it decomposes slowly into potassium chloride and oxygen:

Preparation method
A grinded mixture of potassium chlorate and manganese (IV) oxide, at a ratio of 4:1, is placed in hard glass tube and fitted up as shown in figure bellow. The mixture is then heated and oxygen gas is readily given off. The gas is collected over water. Oxygen has almost the same density as air, so it cannot be collected by the upward displacement of air. It is possible to collect it by downward displacement of water as shown in the figure because it is only slightly soluble in water.
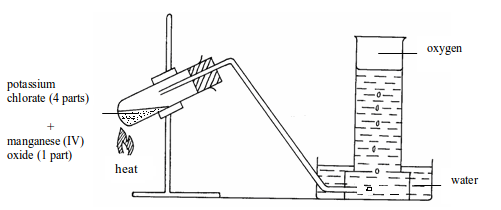
Apparatus for laboratory preparation of oxygen from potassium chlorate
Test for oxygen
Oxygen rekindles a glowing splint of wood. No gases behave like this except dinitrogen oxide, NO2, from which oxygen can be distinguished by the following properties:
1. Oxygen has no smell but dinitrogen oxide has a sweet, sickly smell.
2. When heated with nitrogen monoxide, oxygen produces brown fumes of nitrogen dioxide.
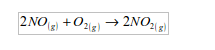
Dinitrogen oxide has no effect on nitrogen monoxide.
Simple Experiments to Demonstrate Properties of Oxygen Gas
Perform simple experiments to demonstrate properties of oxygen gas
1. Action of oxygen on metals
The manner in which oxygen reacts with metals is summarized in the list below.

Reaction with specific metals
Sodium
When burnt in excess of oxygen, sodium burns with an intense yellow flame to give sodium peroxide.
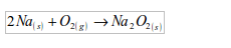
The product is a yellow solid which dissolves in water to give an alkaline solution.
Calcium
The metal burns in air with a red flame giving a white solid of calcium oxide:
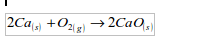
Magnesium
Magnesium burns with a brilliant white flame, leaving a white ash of magnesium oxide:
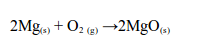
Iron
Iron burns in air with a shower of sparks leaving a brown-black solid of triiron tetraoxide:
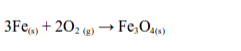
Copper
Copper burns in a stream of oxygen to give a black solid of copper (II) oxide:
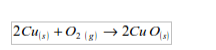
In general, metals react with oxygen to form basic oxides.
Action of oxygen on non-metals
Carbon
Red-hot carbon combines vigorously with oxygen to form carbon dioxide, giving no residue:
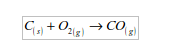
Sulphur
Sulphur burns with a blue flame giving misty white fumes of sulphur dioxide:
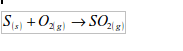
Phosphorus
Phosphorus bursts into flame in air or oxygen, without being heated (that is why it is stored under water). A white solid, phosphorus pentoxide is formed.
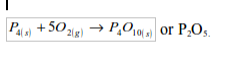
Properties of Oxygen
Explain properties of oxygen
Physical properties
- It is a clear, colourless gas with no smell.
- It is a neutral gas (it is neither basic nor acidic in character)
- It is slightly soluble in water (100 cm3 of water at room temperature dissolves about 4 cm3 of oxygen).
- It has almost the same density as water although slightly denser than air. 5. It boils at -183ºC and freezes at -218ºC.
Chemical properties
- Oxygen supports combustion
- It is a very strong oxidizing agent.
- Oxygen is very reactive. It reacts vigorously with a great many metals and non-metals to form basic and acidic oxides respectively. Metal + Oxygen gives metallic oxide (most of these are basic in character). Non-metals + Oxygen gives non–metallic oxide (most of these are acidic in character).
Uses of Oxygen
Uses of Oxygen in Daily Life
List uses of oxygen in daily life
includes
- The oxygen in the air and that dissolved in water and soil is used by all respiring organisms. Also all types of burning need oxygen.
- It is used in the oxyacetylene (oxygen–ethyne) flame for welding and cutting steel.
- It is extensively used for removing impurities from pig iron in order to produce steel. Oxygen is blown into molten iron to remove impurities such as carbon or phosphorus, which are expelled in the form of gases, i.e. their oxides.
- Oxygen is used as an aid to breathing in hospitals, high altitude climbing or flying, and in deep sea diving.
- Liquid oxygen is used in the burning of fuels such as kerosene, hydrogen and hydrazine used in various types of rockets.
- It is used in the L-D process for making steel.
Relationship between Some Uses of Oxygen to its Properties
Relate some uses of oxygen to its properties
There is relationship between uses of hydrogen and its properties. For example, oxygen is used as an aid to breathing in hospitals and at extreme altitudes because it supports life, and for combustion because it supports burning. Likewise, due to its highly reactive nature, oxygen is used for removal of impurities, welding, in the L-D process for making steel, and in burning of fuels in rockets.







If possible sample questions involving in this topic
ReplyDelete