Join Our Groups
TOPIC 4: THE SCIENTIFIC PROCEDURE
Significance of scientific procedure
The Concept of Scientific Procedure
Explain the concept of scientific procedure
The scientific method (procedure) is a process that scientists use to ask questions and conduct investigations to find answers to these problems. It is a logical approach to problem solving by observing and collecting data, formulating hypotheses, testing hypotheses, and formulating theories that are supported by data. The scientific method provides a standardized way for scientists to conduct their work. However, many scientists work according to other methods as well.
The Importance of the Scientific Procedure
Explain the importance of the scientific procedure
Includes
- The scientific procedure makes a researcher or an experimenter more systematic and organized when investigating or solving a problem.
- It gives a means by which one can get a solution to several questions about natural phenomena, e.g. why does water expand when it freezes?
- It may lead to discoveries and innovations.
- Provides background knowledge upon which future references may be made.
- It makes our sense organs more effective in exploring our natural world. That is, we become more sensitive to environmental changes.
- It makes us use the available resources more sustainably in solving everyday problems.
- Assists us in predicting the future outcome based on the present condition.
- Assists us in testing the validity or the possibility of an event, phenomenon or problem.
The main steps of the scientific procedure
Each Step of the Scientific Procedure
Describe each step of the scientific procedure
Observation (identification) or statement of the problem
The first step of the scientific procedure is to identify a researchable problem. A problem is an obstacle that makes it difficult to achieve a desired goal, objective or purpose. It refers to a situation, condition or issue that is unresolved. Observation refers to identification of a chemical phenomenon. This may include observing the colour, smell, texture of a substance, and so on. Observing involves the use of senses to obtain information. Observation is more than the bare fact of observing. It is determined by use of five senses namely, smell, touch, taste, vision and hearing. For example, to identify the colour of a substance you have to see it with your eyes. The same case applies to detection of the smell of a substance or gas produced by reacting substances in a laboratory. To be able to detect the smell of a gas you have to use your nose to smell it.
Observation helps a scientist to identify a problem. Observation may involve making measurements and collecting data. The data may be descriptive (qualitative) or numerical (quantitative) in nature. Numerical information such as the fact that a sample of sulphur powder measures 50g is quantitative. Non-numerical information, such as the fact that the colour of anhydrous copper (II) sulphate is white, is qualitative.
Once you identify a problem, it becomes easy to state it scientifically. For example, you can observe that when you put a given volume of water in a narrow container and expose it to open air, it takes much longer to evaporate and decrease in volume. However, when you put the same amount of water in a wide container, it takes a much shorter time to do so. This phenomenon can be investigated scientifically.
Hypothesis formulation
After identifying and stating the problem, you can formulate a testable hypothesis for that problem. A hypothesis is a statement. It is a prediction or proposed solution to a problem based on prior knowledge or known information about a chemical phenomenon. It is a logical guess about the outcome of the experiment. A hypothesis must be able to be tested. Therefore, a hypothesis can be described as a tentative explanation for an observation, phenomenon, or scientific problem that can be tested by further investigation. It can be rejected, modified, or accepted only after conducting an experiment to prove or disprove it.
Let us take an example of water at the previous stage. It was observed that the water held in a wide container evaporated faster than that in a narrow container. Based on what we know about evaporation (prior knowledge) we can formulate a hypothesis pertaining to this phenomenon. It is well known that one of the factors affecting the rate of evaporation is the surface area. From this fact, we can formulate a testable hypothesis which states that “evaporation of water increases with increase in surface area of the container in which that water is placed”. This is just a statement. It can be proved wrong or correct by setting up and doing an experiment. Remember that this is just an example, though not very much related to chemistry. We can turn to another relevant example as well.
Now, let us look at an example of anhydrous copper (II) sulphate. The anhydrous salt is in powder form. When you expose this salt to open air, it changes its colour and shape, from its original white powder to blue crystals. Why does this happen? From our knowledge of the properties of this salt (prior knowledge or information gathered) when it is placed in open air, it absorbs water vapour from the air. It is this water vapour which it absorbs that turns it blue. We can go as far as formulating a hypothesis, which states that "When white anhydrous copper (II) sulphate powder is exposed to open air, it absorbs water vapour from the air and turns into blue crystals". We still have a doubt about this hypothesis. How do we know that the liquid absorbed by the salt is really water? To accept or reject this hypothesis, we must conduct an experiment.
Experimentation
After making a hypothesis, the next step is to plan and conduct an experiment. Planning an experiment involves writing down steps for an experiment that will answer the question. It should be remembered that experimental plan should include short and clear steps. It should also include the materials and methods that will be used in the experiment. These may include safety gears such as goggles, gumboots, gloves, etc. It must also state all expected hazards to be accompanied with the reacting substances or chemical phenomena being experimented. This could either occur as a result of mishandling chemicals or apparatus, improper experimental procedure or even testing the products obtained from the experiment.
In the scientific method, an experiment is a set of observations (qualitative or quantitative) made in the context of solving a particular problem or question. An experiment is conducted in order to retain or falsify a hypothesis concerning a particular phenomenon. The experiment is a basis in the practical approach to acquiring deeper knowledge about the chemical world.
Experimenting involves carrying out a procedure under controlled conditions in order to make observations and collect data. To learn more about matter, chemists study systems. A system is a specific portion of matter in a given region or space that has been selected for study during an experiment or observation. When you observe a reaction in a test tube, the test tube and its contents form a system.
Your experiment tests whether your hypothesis is true or false. It is important for your experiment to be a fair test. You conduct a fair test by making sure that you change only one factor at a time while keeping all other conditions the same (constant). These factors are also called variables. They are the factors that affect the problem you want to investigate. They can change or be changed during the experiment. Such factors include temperature, volume, speed, light, concentration, light, etc.
Students conducting an experiment
There are three types of variables. These are:
- Dependent variable: This is the factor that changes its value when the values of the other variables change. It is the value being measured.
- Independent variable: This is the factor that is manipulated so as to obtain different values.
- Controlled (or constant) variable: This is the factor that does not change, or is kept constant all the time. It does not affect the result of the experiment.
For example, you might be interested to carry out an experiment to determine the influence of the concentration of phosphorus fertilizer on maize growth. To get the best results, you grow maize in similar conditions of soil and atmospheric environment (controlled variable) but vary the quantity of fertilizer in each test (independent variable). Then you measure the height of maize plants (dependent valuable) after a certain interval of time as shown in figure below. The value of the height you will obtain will obviously depend on the amount (concentration) of the fertilizer applied. This is a typical fair test. However, most chemistry experiments do not involve fair tests.
Now, let us turn back to our experiment. In the example of determining whether the surface area increases the rate of evaporation or not, we can design an experiment to prove or disprove this phenomenon. This is conducted by filling a basin (with large surface area) and a bucket (with small surface area) with 10 litres of water each. Then the two containers are placed in open air for 3 days. Here, care must be taken to place both containers under similar environmental conditions. Containers must be of the same type, that is, both must be plastics, metals, etc. In addition, the water used must be obtained from the same source.
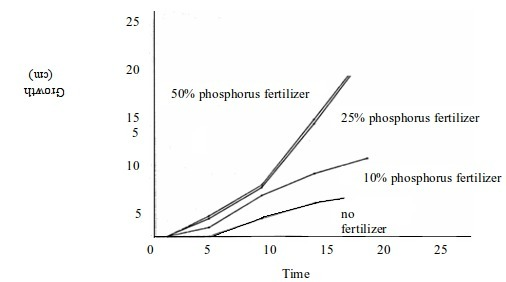
The effect of fertilizer on plant growth
The only variable to be kept constant is the volume of water, which is set to the volume of 10 litres. You should repeat your experiment several times to make sure that the first results were not just an accident.

Determination of the effect of surface area on the rate of evaporation of water
Observation and collection of data
Observation and recording of data must be done from the beginning to the end of the experiment. Data is the information gathered during the experiment. This can include descriptive (qualitative) and numerical (quantitative) data. Numerical data is that which can be measured, for example, 10 litres of water, 5g of copper, a five centimetre long ribbon of magnesium, etc. Qualitative data include information that cannot be measured, e.g. colour, shape or appearance, smell, feel, etc. Recording data is an important part of the scientific method because it helps scientists organize their ideas and observations. Charts, graphs, lists, diagrams, tables and even sketches are all the ways of recording data during experiments. Records appearing in the form of tables are easy to read, understand and interpret.
Continuing with our hypothetical experiment for determining the effect of surface area on the rate of evaporation, we expect that at the end of the experiment the volume of water in each container will have dropped to a certain extent.
We also expect that the volume of water lost from the basin will be bigger than that lost from the bucket. This means that more water will evaporate from the basin than from the bucket. Considering that scenario, we can then predict what the data can be like. Let us take this model as a real experiment and assume the kind of results that could be observed and collected during the experiment.
After 3 days of the experiment, water from each container was measured. The results obtained were summarized in the following table.
Source: hypothetical
Data collected from evaporation experiment after 3 days
| Amount of water | Type of container | |
| Basin | Bucket | |
| Initial volume Final volume | 10 litres 7.0 litres | 10 litres 8.5 litres |
| Amount of water lost (evaporated off) | 3.0 litres | 1.5 litres |
Data analysis and interpretation
Once your experiment is complete and after you have collected data, you analyse your data to see if your hypothesis is true or false. In table 4.1, we find that, at the end of the experiment, 3 litres of water had evaporated off from the basin as compared to 1.5 litres from the bucket. What does this data tell us? What is it trying to reveal? This means that from the basin (with large surface area) water evaporated faster than that from the bucket (with small surface area). The data reveals the fact that surface area plays a major role in evaporation of water and many other liquid substances.
However, you may sometimes get unexpected results. You may find that your hypothesis was false. In such a case, you will construct a new hypothesis and start the entire process of the scientific method over again. Even if you find that your hypothesis was true, you may want to test it again in a new way.
Conclusion
The last stage (at this level of study) of the scientific method is to make inferences and draw a conclusion. Scientists look at the information they gathered and observed. Then they make connections to draw a conclusion. These conclusions may be or may not be in agreement with their predictions. Scientists make incorrect predictions all the time. An important part of the scientific process is to understand why predictions were incorrect. Many scientists will repeat an experiment several times to see if they can replicate the results before concluding. This ensures that they have conducted the experiment the same way each time and make sure no introduced errors or outside factors affected the experiment's outcome.
Based on data in a hypothetical experiment above, we found that 3.0 litres of water evaporated from the basin (a container with wide mouth and hence a large surface area). At the same time, 1.5 litres of water evaporated from a bucket (a container with a narrow mouth and hence a small surface area). From this result, of course, we can conclude that evaporation of water increases with increase in surface area of the container in which that water is kept. Therefore, our hypothesis is proved true and correct. Remember always to base your conclusion on the collected and analysed data, though it may deviate, to some extent, from the reality for one reason or another.
ADDITIONAL NOTES ON SCIENTIFIC PROCEDURE
In advanced study, the last step of the scientific method is to share what you have learned. Scientists share information so that others can use the findings to create different questions and conduct different experiments. Sharing information is an important part of working together. Professional scientists will publish their final reports in scientific journals, magazines, books, or even present their results at scientific conferences. For the purpose of study at this level, the last step is conclusion.
Even though we show the scientific procedure as a series of steps, keep in mind that new information or thinking might cause a scientist to back up and repeat steps at any point during the process. A process like the scientific method that involves such backing up and repeating is called an iterative process. Therefore, the scientific process is an iterative process.
Application of the scientific procedure
The scientific procedure is used in many areas and in different fields of study. It is especially applied by scientists and researchers to find solutions to various scientific problems. Below are some of the areas where the scientific procedure is applied:
- In scientific research: Researchers normally apply the scientific method when conducting researches on diverse scientific problems or phenomena. A researchable problem whose solution is sought for without following the correct sequence of the steps of the scientific method is not likely to get resolved.
- In a field study: A field study (or field work) is often conducted to find answers to problems or test hypotheses. It involves doing some practical work that applies the scientific methods.
- When conducting experiments: An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the accuracy of a hypothesis. Experiments vary greatly in their goals and scale, but always rely on repeatable procedure and logical analysis of the results.
- In project work: A project is a planned piece of work that involves careful study of a subject or problem over a period of time, so as to find information on the subject or problem.
The Scientific Procedure to Carry Out Investigations in Chemistry
Use the Scientific procedure to carry out investigations in chemistry
In this chapter, we have used two major examples to explain the concept of experimental procedure in detail. These are the rate of evaporation of water and exposure of anhydrous copper (II) sulphate powder to open air. For easy understanding and quick reference by students, the two examples are summarized below. Note that the test for the anhydrous copper (II) sulphate powder was not explained in full. However, the summary can give you a good picture on how to go about experimenting it.
A. The rate of evaporation of water
Steps
- Problem/question: Does surface area affect the rate of evaporation of water?
- Hypothesis: Evaporation of water increases with increases in surface area
- Experimentation: A basin and a bucket are filled with 10 litres of water each. They are left exposed to open air, under similar conditions for a period of 3 days.
- Observation and data collection: After 3 days, the remaining water in containers was measured carefully. The results were recorded in a table.
- Data analysis and interpretation: It was found that 3 litres of water had evaporated from the basin and 1.5 litres from the bucket. From this data, it was discovered that much water (3 litres) had evaporated from a container with large surface area (basin) as compared to only 1.5 litres of water that had evaporated from a container with a small surface area (bucket).
- Conclusion: Since a large amount of water evaporated from the basin as compared to that from the bucket, it is correct to conclude that surface area affects the rate of evaporation of water and that the larger the surface area the higher is the evaporation. Therefore, the hypothesis is proved to be true.
B. Exposure of anhydrous copper (II) sulphate powder to open air
Steps
- Problem/question: Why does anhydrous copper (II) sulphate powder change into hydrated blue crystals when exposed to open air?
- Hypothesis: When exposed to open air, the anhydrous copper (II) sulphate powder absorbs water vapour from the air and this water vapour turns it to blue crystals.
- Experimentation: The anhydrous sulphate is exposed to open air to absorb sufficient water vapour. Then the hydrated sulphate is heated to drive out all the liquid in it.
- Observation and data collection: The sample of hydrated blue crystals loses the liquid in it and turns to its original white powder. The vapour given off is carefully collected, cooled down to liquid, and then put in a beaker or test tube.
- Data analysis and interpretation: The collected liquid is subjected to various water tests to justify whether it is water or just the other liquid substance. The liquid is identified as water.
- Conclusion: The anhydrous copper (II) sulphate was exposed to air only. We also know that air contains water vapour. Because of this reason, it is correct to conclude that the water came from the water vapour contained in air. The water turned the white powder to blue crystals. Therefore, our hypothesis is true.







EmoticonEmoticon